周围神经损伤
-
Figure 1|TERT expression in K?lliker’s organ in postnatal cochlea.
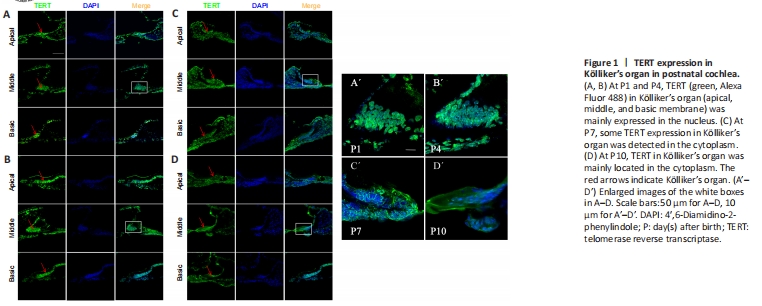
To examine TERT in the developing cochlea before hearing onset early after birth, we performed immunofluorescence staining for TERT in frozen sections of mouse cochlea at P1, P4, P7, and P10 after birth, TERT was expressed in apical, middle, and basal membranes, mainly in K?lliker’s organ (Figure 1A). TERT was localized in the nucleus of K?lliker’s organ at P1 and P4 (Figure 1A, B, A’, and B’). At P7, some TERT expression was detected in the cytoplasm (Figure 1C and C’). At P10, TERT was primarily located in the cytoplasm (Figure 1D and D’). We also observed K?lliker organ degeneration during this period; at P4, K?lliker supporting cells of the basic turn decreased (Figure 1B), and the middle turn and apical turn began to degenerate at P7 (Figure 1C) and P10 (Figure 1D).
Figure 2|Morphological changes of supporting cells in vitro.
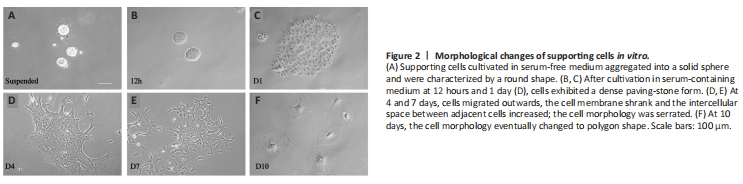
To further examine the potential function of TERT in supporting cells, we isolated and cultured cochlear supporting cells in vitro. The morphology of supporting cells gradually changed as the culture period progressed. Before adherent growth, supporting cells cultured in serum-free cell culture medium aggregated into a solid sphere (Figure 2A). We then cultured cells in cell culture medium containing calf serum, and the cells gradually adhered to the bottom of the cell plate (Figure 2B–F). At 12 hours and 1 day, the cells showed a dense paving-stone form (Figure 2B and C). The cells then migrated outwards; as the cell membrane shrank, the intercellular space between adjacent cells increased, and the morphology became serrated (Figure 2D–F). On day 10, the cell morphology eventually changed to a polygonal shape (Figure 2F).
Figure 3|TERT expression in K?lliker’s organ supporting cells in vitro.
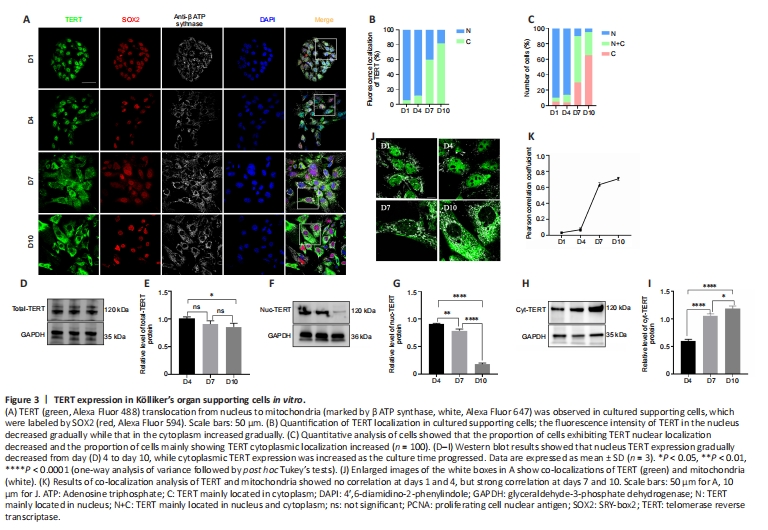
We next examined the expression of TERT in cultured supporting cells in vitro to determine whether TERT showed the same translocation pattern. The results revealed a TERT translocation tendency in postnatal cochlear supporting cells in vitro similar to that observed in vivo (Figure 3A). Semi-quantitative analysis of TERT localization revealed that cytoplasmic TERT gradually increased from day 1 to day 10 (Figure 3B). To further analyze the TERT translocation trends in supporting cells in vitro, we scored over 100 supporting cells on the basis of TERT expression: higher in the nucleus, evenly distributed between the nucleus and cytoplasm, or higher in the cytoplasm. TERT was predominantly localized in the nucleus on days 1 and 4 (95.4% and 93.4% of cells, respectively), and more TERT signal was detected in the cytoplasm on day 7 (20%, 29.6%, and 50.5% of cells showed nuclear, cytoplasmic, and pan-cellular TERT distribution, respectively; Figure 3C). TERT was mainly localized in the cytoplasm on day 10 (95.3% of cells; Figure 3C).
To further confirm the translocation of TERT, we extracted total protein, cytoplasmic protein, and nuclear protein of cochlear supporting cells and performed western blotting. TERT was stably expressed on days 4, 7, and 10 in supporting cells in vitro (Figure 3D and E). The amount of TERT in the nucleus gradually decreased over time while the amount of TERT in the cytoplasm gradually increased (Figure 3F–I).
To examine whether TERT localized to mitochondria, mitochondria were examined using ATP synthase beta antibody (Figure 3J). Analysis of colocalization results revealed no correlation of TERT and mitochondria on days 1and 4 (Figure 3K; r = 0.03 ± 0.01 and r = 0.07 ± 0.02, respectively), but a strong correlation on days 7 and 10 (Figure 3K; n = 5; r = 0.63 ± 0.03 and r = 0.71 ± 0.02, respectively). These data indicate that in postnatal cochlear supporting cells in vitro, TERT shifts from the nucleus to the cytoplasm and localizes to the mitochondria.
Figure 4|Changes in mitochondrial function in cochlear supporting cells in vitro.
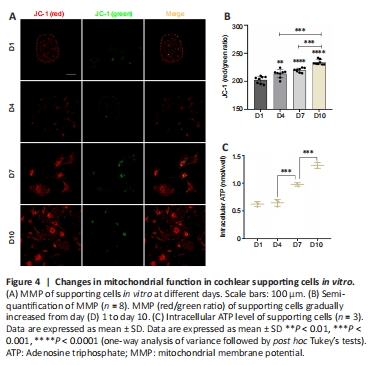
Mitochondria are fundamentally involved in maintaining normal cell function (Haider et al., 2021). Normal MMP is a prerequisite for maintaining mitochondrial oxidative phosphorylation and ATP production (Audi et al., 2020; Haider et al., 2021), and the stability of the MMP is conducive to maintaining the normal physiological functions of cells (Haider et al., 2021). Hence, MMP is an important indicator of mitochondrial function. To investigate the functional status of mitochondria in supporting cells during TERT translocation, we detected MMP levels using immunofluorescence. The results revealed that the MMP of supporting cells on days 7 and 10 gradually increased compared with that on days 1 and 4 (Figure 4A and B).
Figure 5|Detection of ATP release and intracellular Ca2+ transients in cochlear supporting cells in vitro.
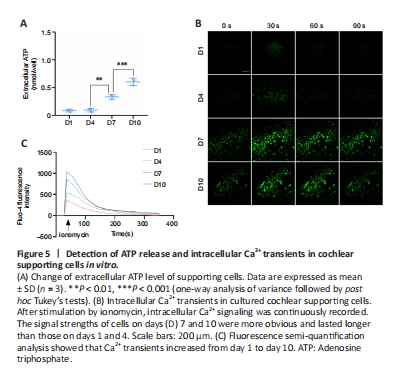
Spontaneous electrical activity in the cochlea originates from the periodic release of ATP (Tritsch et al., 2007). When ATP is released from an intracellular source, it acts as a fast-acting intercellular messenger (Peng et al., 2012). Extracellular ATP plays a central role in driving the Ca2+ waves that coordinate the activity of inner hair cells (Tritsch and Bergles, 2010). To quantitatively investigate ATP release from cochlear supporting cells during TERT translocation, we measured the extracellular concentration of ATP. The results revealed that the extracellular ATP concentration of supporting cells increased on days 7 and 10 compared with levels on days 1 and 4 (Figure 5A).
Ca2+ is an important signal molecule of intercellular communication in the cochlea and is involved in a large number of signaling pathways. Increased intracellular free Ca2+ can initiate the opening of half channels. In the developing cochlea, the intercellular Ca2+ waves are elicited by ATP release that affects inner hair cell activity (Tritsch et al., 2007; Anselmi et al., 2008; Majumder et al., 2010). In the acutely isolated cochlea, spontaneous Ca2+ elevations were observed within inner supporting cells of K?lliker’s organ before the onset of hearing (Tritsch and Bergles, 2010). These Ca2+ transients became more prominent, with the frequency and mean area increasing from early prehearing (P0–1) to late prehearing (P8–10) stages (Tritsch and Bergles, 2010). To explore the changes in Ca2+ levels in supporting cells during TERT translocation, we detected the intracellular Ca2+ concentration. The results revealed different Ca2+ transients in postnatal cochlear supporting cells in vitro on different days. After ionomycin stimulation, intracellular Ca2+ signaling of cells on different days was detected at 30 seconds. The signal strengths of cells on days 7 and 10 were more apparent than those on days 1 and 4 (Figure 5B). Semi-quantitative analysis of Ca2+ signaling revealed that the amplitudes on days 7 and 10 (mean Fluo-4 fluorescence intensity, 830.73 ± 30.63 and 1007.23 ± 25.12) were higher than those on days 1 and 4 (327.31 ± 25.75 and 514.41 ± 26.79; n = 3; D4 vs. D7, P < 0.0001, ordinary one-way analysis of variance; Figure 5C). Moreover, at 60 seconds, Ca2+ signaling on days 1 and 4 decreased (mean Fluo-4 fluorescence intensity, 255.31 ± 18.93 and 427.43 ± 30.01), and the Ca2+ signaling on days 7 and 10 still existed (676.89 ± 18.37 and 820.66 ± 28.38; n = 3; D4 vs. D7, P < 0.0001, ordinary one-way analysis of variance), indicating that Ca2+ waves lasted longer on days 7 and 10 than on days 1 and 4 (Figure 5B and C). These results indicated that Ca2+ transients increased from day 1 to day 10.
Figure 6|Detection of oxidative stress and apoptosis in supporting cells in vitro.
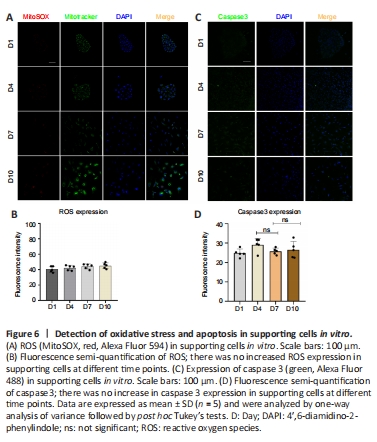
To determine whether TERT relocation in supporting cells in the developing cochlea was induced by oxidative stress or apoptosis, we detected the level of ROS and apoptosis during in vitro culture. The results revealed no increase in ROS (Figure 6A and B) or caspase-3 (Figure 6C and D), and TUNEL assays showed no changes in apoptosis (Additional Figure 2A and B) in supporting cells from day 1 to day 10. This suggests that there is no oxidative stress or apoptosis in supporting cells in vitro from day 1 to day 10, and therefore the TERT translocation into mitochondria was not induced by oxidative stress or apoptosis. These data imply that the role of TERT in mitochondria does not involve non-antioxidant or anti-apoptotic functions.