周围神经损伤
-
Figure 2| Immunofluorescence detection of CD90+ fibroblast cells and S100+ Schwann cells.
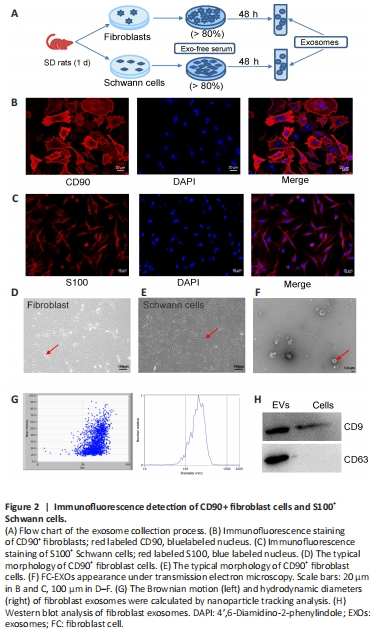
To determine whether exosomes derived from fibroblast and Schwann cells can stimulate axonal regeneration, we purified exosomes from the conditioned medium of fibroblasts and Schwann cells, respectively. As shown in the flowchart in Figure 2A, primary cells were isolated from 1-week-old SD rats, and cultured in vitro. When the cell density reached 80%, the exo-free serum medium was replaced with fresh medium and the cells were cultured for another 48 hours, after which the supernatant was collected to purify the exosomes. Immunofluorescence was used to assess the purity of the cells. The fibroblast cells showed strong positive expression of CD90, with a cell purity of 90% (Figure 2B). Similarly, the Schwann cells showed strong positive expression of S100 and 85% purity (Figure 2C). The CD90+ fibroblasts were shaped like shuttles or irregular triangles, with an oval nucleus in the center and cytoplasmic protrusions that grew radially (Figure 2D). The nuclei of the S100+ Schwann cells were oval, with the long axis parallel to the axon, and there was little cytoplasm around the nucleus (Figure 2E). Thus, we considered the status and density of these two cell types adequate for use in subsequent experiments. Under transmission electron microscopy, FC-EXOs presented a typical round or oval cup-mouth structure (Figure 2F). As shown in Figure 2G, nanoparticle tracking analysis showed that the average size of the FC-EXOs was between 100 and 150 nm. In addition, western blot verified the expression of exosome markers and showed that CD9 and CD63 were significantly expressed in FC-EXOs (Figure 2H). The SC-EXO identification results were consistent with FC-EXO results, suggesting that both types of exosomes could be used for subsequent sequencing analyses.
Figure 8|Rps5 expression in exosomes derived from fibroblasts and Schwann cells in vitro can effectively promote axon regeneration in dorsal root ganglion (DRG) neurons.
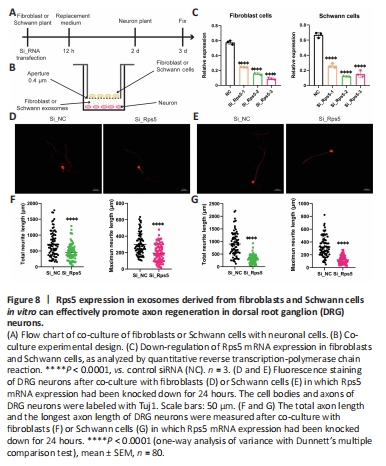
To determine the effect of regulating Rps5 expression in fibroblasts and Schwann cells on axon regeneration, we established an indirect contact co-culture model of fibroblasts or Schwann cells with DRG neurons (Figure 8A and B). First, we transfected fibroblasts and Schwann cells with a gene-specific siRNA to down-regulate Rps5 expression. qRT-PCR analysis showed that, compared with the control siRNA group, the gene-specific siRNA effectively reduced Rps5 expression in fibroblasts and Schwann cells. On the basis of these results, we selected siRps5-3 for further co-culture experiments (Figure 8C). In the co-culture experiment, we measured the axon length of DRG neurons by immunofluorescence 24 hours after indirect contact with fibroblasts or Schwann cells in which Rps5 expression had been knocked down (Figure 8D and E). The average total length of DRG neuron axons in the experimental group co-cultured with fibroblasts was 450.77 μm, and the average of the longest axons on each cellwas 193.42 μm; the average total axon length of DRG neurons in the control group was 711.65 μm, and the average length of the longest axon was 299.22 μm (Figure 8F). The average length of DRG neuron axons in the experimental group co-cultured with Schwann cells was 298.40 μm, and the average length of the longest axons was 123.04 μm; the average total axon length of DRG neurons in the control group was 898.18 μm, and the average longest axon length was 231.76 μm (Figure 8G). In summary, down-regulation of Rps5 expression in fibroblasts or Schwann cells significantly inhibits axon growth in co-cultured DRG neurons.