脑损伤
-
Figure 5| C1q/tumor necrosis factor-related protein-6 (CTRP6) overexpression attenuates ischemia/reperfusion (I/R)-induced inflammatory responses and apoptosis in the brain.
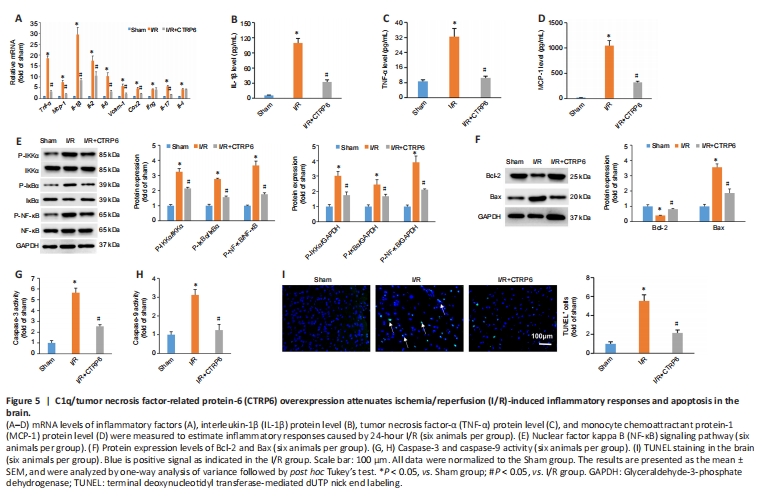
Neuroinflammation plays profound roles in diabetic stroke (Lindsberg and Grau, 2003). We detected an inflammatory response in CTRP6-overexpressing diabetic mice. The mRNA of numerous pro-inflammatory factors, such as Tnf-α, Mcp-1, Il-1β, Il-2, Il-6, Vcam-1, Cox2, Ifng, Il-17, and Il-4 were higher in the I/R group, and these elevations were suppressed by CTRP6 overexpression (Figure 5A). However, CTRP6 did not affect the elevated levels of IL-4 that were observed in the I/R group (Figure 5A). ELISA confirmed the mRNA results. CTRP6 alleviated the pathological accumulation of IL-1β, TNF-α, and MCP-1 that wasfound in the I/R group (Figure 5B–D). As reported, NF-κB regulates several inflammatory genes and plays a key role in cerebral diseases (Wang et al., 2019). CTRP6 supplementation attenuated phosphorylation of IKKβ, IκBα, and NF-κB in the diabetic mice (Figure 5E). Next, we found that apoptosis-related proteins were also affected by CTRP6 treatment. CTRP6 supplementation increased Bcl-2 and decreased Bax in diabetic mice after I/R (Figure 5F). Consistent with these findings, we also observed that CTRP6 overexpression decreased the elevated caspase 3 and caspase 9 activity that was observed in diabetic brains after I/R (Figure 5G and H). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining showed that CTRP6 overexpression significantly reduced the number of TUNEL-positive cells in diabetic brains after I/R (Figure 5I).
Figure 8| C1q/tumor necrosis factor-related protein-6 (CTRP6) cannot protect against ischemia/reperfusion (I/R) injury in sirtuin 1 (Sirt1)-deficient mice.
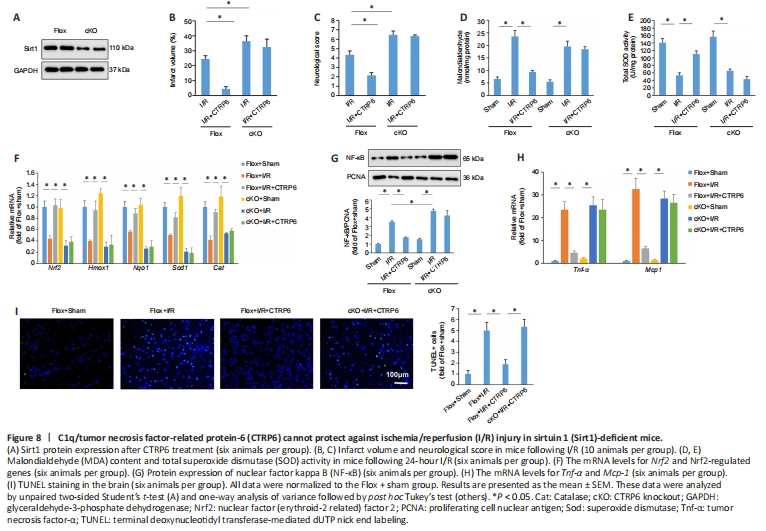
Next, we used mice with neuron-specific depletion of Sirt1 (Figure 8A). CTRP6 supplementation in the absence of Sirt1 could not provide protection against I/R-induced injury, as reflected by infarct volume and neurological deficits (Figure 8B and C). The inhibitory effects of CTRP6 on MDA production were also blocked by Sirt1 deficiency (Figure 8D). In Sirt1-deficient mice, CTRP6 did not increase total SOD activity in the model mice following focal cerebral I/R (Figure 8E). Further analysis of anti-oxidative gene expression confirmed that the inhibitory effects of CTRP6 were blocked by Sirt1 deficiency (Figure 8F). The decreased nuclear NF-κB and inflammatory factors after CTRP6 treatment were also abolished by Sirt1 knockout (Figure 8G and H). Sirt1 knockout also abrogated the anti-apoptotic effects of CTRP6 in the model mice following focal cerebral I/R (Figure 8I).