脑损伤
-
Figure 7|Strategies to promote the development of brain organoids.
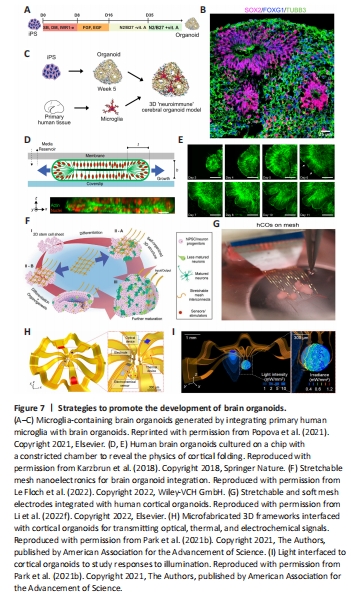
Despite the rapid development of brain organoids and their promising prospects as tools in neuroscience, brain organoid technology has some significant limitations. Although microphysiological systems can be used to optimize the microenvironment of brain organoids, it is infeasible to build human-brain-relevant culture conditions (Trujillo and Muotri, 2018; Tan et al., 2021). Moreover, the induction of brain organoids is mostly based on empirical protocols or previous studies; thus, essential factors could be missing or over-represented, leading to abnormal neural development and maturation and a failure to faithfully produce human brain structures. For example, immune cells (e.g., microglia and astrocytes) and endothelial cells do not appear during ectoderm induction, leading brain organoids to lack an immune system and vasculature. Therefore, the optimization of cell cultures with selected factors and media is vital in the generation of advanced brain organoids. There is no doubt that brain-resident microglia play critical roles in brain development and microenvironmental maintenance. Recently, neuroimmune organoids have been generated by co-culturing brain organoids with primary or iPSC-derived microglia (Popova et al., 2021; Figure 7A–C). The presence of the microglia was found to protect against double-stranded DNA breaks and promote neural network synchronization in brain organoids by modulating synaptic density. Alternatively, gene editing, such as PU.1 (myeloid-specific transcription factor) overexpression, can be used to produce microglia-containing human brain organoids, which has facilitated studies of brain development and diseases (Cakir et al., 2022; Zhang et al., 2023a). Blood vessels are independent structures that provide adequate oxygen and nutrients and a structure for oriented neuron growth. Advanced biotechnologies and methodologies, such as genome editing, coculture, multi-differentiation, microfluidic chips, transplantation in vivo, and assembly methods, have been applied to promote neural organoid vascularization (Mansour et al., 2018; Cakir et al., 2019; Ham et al., 2020; Kaushik et al., 2020; Shi et al., 2020; Worsdorfer et al., 2020; Yue et al., 2020; Li et al., 2023a; Sun et al., 2022; Wang et al., 2023b). However, the vascular networks generated within organoids are disordered and fail to recapitulate the function of blood vessels. The principle of angiogenesis during neurogenesis needs to be considered for brain organoid vascularization.
Cortical organoids attract significant interest in brain organoid research, as the human cortex is the most complex and evolutionarily expansive part of the brain compared to that of animals (Qian et al., 2019; Rakic, 2009). However, current cortical organoids are still not close to recapitulating the complicated human cerebral cortex. A major limitation of these systems is that the cortical wrinkling seen in the human brain does not occur in cortical organoids. One of the most prominent characteristics of the cortex is the cortical expansion and folding that lead to the size and complexity of the human brain (Fernandez et al., 2016; Borrell, 2018; Llinares-Benadero and Borrell, 2019). Previous evidence has shown that genetic, cell biological, and biomechanical factors are critical in the complex and multifaceted process of cerebral cortical folding (Albert and Huttner, 2015; Florio et al., 2015; Del Toro et al., 2017; Borrell, 2018; Llinares-Benadero and Borrell, 2019). For instance, Jaenisch’s group showed that key features of the developing human cortex, including its expansion in size and surface folding, can be modeled in brain organoids by activating the PTEN-AKT signaling pathway (Li et al., 2017b). However, this evidence inadequately reflects the gene expression associated with normal human fetal brain development. Moreover, Karzbrun et al. (2018) reported the appearance of surface wrinkles in developing human cerebral organoids on a chip (Figure 7D and E). The physical mechanism modeled cortex folding extremely well. Nevertheless, the physical cues involved in human brain development are still poorly understood and need to be comprehensively assessed in follow-up studies (Budday et al., 2015). Previous studies provided evidence that oRGCs were largely absent in lissencephalic rodents, despite being important for human cortical expansion, suggesting that brain gyrification could be closely associated with oRGCs (Bershteyn et al., 2017; Rash et al., 2019; Zarzor et al., 2023). Furthermore, it is worth mentioning that cortical organoid maturation resembles the developmental stages of the human brain and plays a vital role in the occurrence of gyrification because the folding process takes place during gestational weeks 16–40 (Sun and Hevner, 2014; Garcia et al., 2018a, b; Urresti et al., 2021). Therefore, more novel, sophisticated, and appropriate designs and methodologies should be implemented to create human brain organoids with gyri and to recapitulate the properties of native brains in vitro.
Although previous studies have detected neuronal action potentials, excitatory and inhibitory postsynaptic currents, and spontaneous network activity within neural organoids, single-neuron action potentials have not be recorded (Mansour et al., 2018; Trujillo et al., 2019; Tasnim and Liu, 2022). Recently, Le Floch et al. (2022) designed stretchable mesh nanoelectrodes that, when distributed across brain organoids, created a cyborg brain organoid platform (Figure 7F). The integrated, stretchable electrode arrays enabled long-term stable, continuous recording and captured the emergence of single-cell action potentials within brain organoids during early development. Semiconductor devices can be used to not only provide promising platforms for evaluating the functional development of brain organoids but also to introduce potential stimulators, such as electronic, optoelectronic, thermal, mechanical, and biochemical interfaces, to promote brain organoid development (Park et al., 2021b; Le Floch et al., 2022; Li et al., 2022e; Figure 7G–I). Given the critical roles of electrical stimulation in neurogenesis and neurodevelopment, exogenous electrical stimulation from biocompatible meshes was used to monitor and modulate the electrical activity of neurons with cortical organoids. Moreover, the rapid development of advanced biomaterials, such as conductive hydrogel polymers, opens new avenues for the building of 3D electroconductive scaffolds for brain organoids (Xu et al., 2020; Wang et al., 2021a; Song et al., 2022).