神经损伤与修复
-
Figure 2|PERK activation specifically in mature oligodendrocytes induced myelin thinning in the adult CNS.
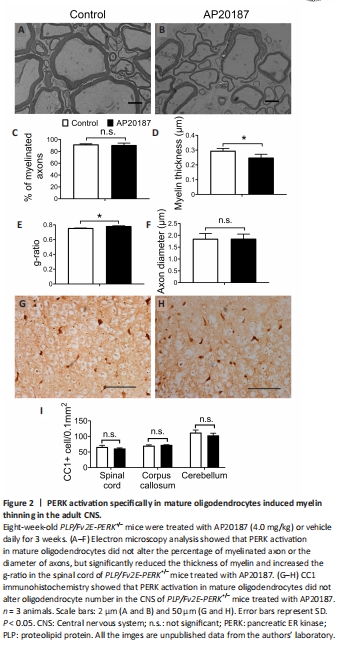
Evidence suggests that the impact of PERK activation on cell viability and function is activity-dependent and/or context-dependent (Pakos-Zebrucka et al., 2016; Lin and Stone, 2020). Previous studies have suggested that PERK activation in mature oligodendrocytes can lead to progressive myelin thinning in the adult CNS of mice with Sel1L deletion in oligodendrocytes by inhibiting myelin protein translation (Wu et al., 2020; Wu and Lin, 2023). However, these studies cannot rule out the possibility that the unique cellular context of Sel1L-deficient oligodendrocytes is necessary for the myelin thinning induced by PERK activation in these mice. A prior report revealed that short-term, artificially strong activation of PERK in mature oligodendrocytes results in moderate myelin loss, a reduction in myelin thickness, a reduction in axon diameter, but no oligodendrocyte loss in the CNS of adult mice (Lin et al., 2014a). Given that myelin thickness is known to be influenced by axon diameter (Baumann and Pham-Dinh, 2001; Aggarwal et al., 2011; Stadelmann et al., 2019), this previous report cannot exclude the contribution of the reduction in axon diameter to the reduction in myelin thickness caused by strong PERK activation in mature oligodendrocytes. We have generated PLP/Fv2E-PERK transgenic mice that express the Fv2E-PERK transgene specifically in oligodendrocytes in the CNS (Lin et al., 2013). This transgene, Fv2E-PERK, is an engineered version of the PERK protein, achieved by fusing the eIF2α kinase effector domain of PERK with a polypeptide that incorporates two modified FK506 binding domains (Fv2E) (Lu et al., 2004b). The functionality of Fv2E-PERK is tightly regulated by the FK506 dimerizing agent AP20187, independent of the ER stress signaling (Lu et al., 2004b). We have demonstrated that treatment with AP20187 induces the activation of the PERK pathway specifically in oligodendrocytes of PLP/Fv2E-PERK mice, and this activation exhibits a dose-dependent response (Lin et al., 2013, 2014a, b). To ascertain the role of PERK activation in mature oligodendrocytes in regulating myelin thickness in the adult CNS, we utilized 8-week-old heterozygous PLP/Fv2E-PERK (PLP/Fv2E-PERK+/–) mice and treated them with a medium dose of AP20187 (4.0 mg/kg) or vehicle daily for 3 weeks to moderately activate the PERK pathway in mature oligodendrocytes. AP20187-treated PLP/Fv2E-PERK+/– mice did not exhibit any noticeable neurological symptoms. Importantly, electron microscopy analysis showed that AP20187 treatment did not significantly alter the percentage of myelinated axons or the diameter of axons, but significantly decreased the thickness of myelin and increased the g-ratio in the CNS of PLP/Fv2E-PERK+/– mice (Figure 2A–F). CC1 immunohistochemistry showed that AP20187 treatment did not significantly change oligodendrocyte numbers in the CNS of PLP/Fv2E-PERK+/– mice (Figure 2G–I). Moreover, immunoprecipitation-surface sensing of translation (IP-SUnSET) analysis (Wu et al., 2020; Wu and Lin, 2023) showed that AP20187 treatment significantly reduced the levels of newly synthesized myelin basic protein and PLP in the CNS of PLP/Fv2E-PERK+/– mice (Figure 3). These results demonstrate that PERK activation alone in mature oligodendrocytes is sufficient to induce thinning of originally-existed myelin, without altering axon diameter, in the CNS of adult mice under physiological conditions by suppressing myelin protein translation. Conversely, the precise molecular mechanisms by which PERK activation in mature oligodendrocytes regulates protein biosynthesis and myelin thickness in the adult CNS are not fully understood and warrant further investigation.