神经退行性病
-
Figure 1|Citri Reticulatae Semen extract (CRSE) rescues the viability of amyloid-beta (Aβ1–42)-induced PC12 cells.
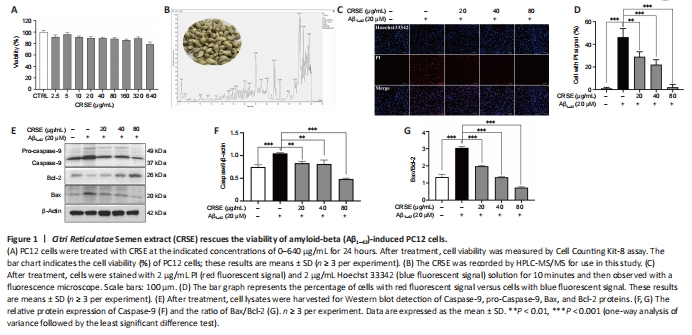
CCK-8 assays were adopted to assess the viability of PC12 cells upon CRSE treatment from 0 to 80 μg/mL. As shown in Figure 1A, no obvious change in cell viability was observed in PC12 cells treated with different concentrations of CRSE. To analyze the chemical profile of CRSE, high-performance liquid chromatography-mass spectrometry (HPLC-MS) chromatogram measured at 254 nm is presented in Figure 1B and with the major components listed in Additional Table 1. Upon Aβ1–42 aggregate induction, the protective effect of CRSE on Aβ1–42-induced cell death was investigated. In Figure 1C and D, while Hoechst 33342 staining showed the total number of cells, propidium iodide (PI) staining indicated the number of dead cells. With the escalating concentration of CRSE treatment from 0 to 80 μg/mL, a decreasing percentage of dead cells stained by PI. This result was further validated by evaluating the protein ratio of apoptotic protein pro-Caspase 9/Caspase 9 and Bax/Bcl-2 in PC12 cells, confirming the protective role of CRSE in the Aβ1–42-induced cell death model in vitro (Figure 1E–G).
Figure 2|Citri Reticulatae Semen extract (CRSE) enhances autophagy induction in cells and Caenorhabditis elegans (C. elegans).
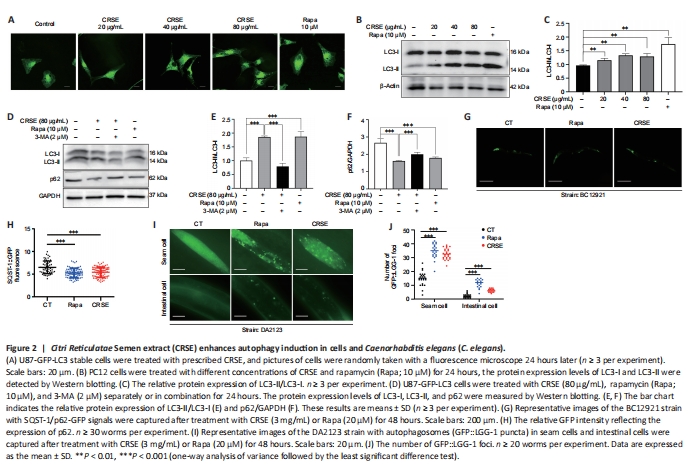
Autophagy can be protective against neurotoxicity as an adaptive strategy (Tran and Reddy, 2021). To confirm the pharmacological role of CRSE, U87-GFP-LC3 stable cells were adopted to evaluate the autophagic effect of CRSE (0 to 80 μg/mL) for 24 hours. Autophagosomal LC3-II is a key marker of autophagic activity; therefore, the level of LC3-II was detected by both immunofluorescence and immunoblotting analysis after CRSE treatment. As shown in Figure 2A, the percentage of cells with puncta formation was increased significantly in a dose-dependent manner after CRSE treatment. Figure 2B and C confirmed that CRSE significantly increased the ratio of LC3-II/LC3-I in PC12 cells. With the addition of 3-MA which inhibits autophagy by altering autophagosome formation, the ratio of LC3-II/LC3-I was decreased with a concomitant increase in the level of the autophagic substrate p62 (Figure 2D–F). To determine whether CRSE induces autophagy in vivo, the transgenic worm strain BC12921 carrying the SQST-1/p62-GFP fusion protein and DA2123 strain expressing GFP::LGG-1 were employed. As shown in Figure 2G–J, a significant reduction in the expression of SQST-1/p62-GFP and an increased number of GFP::LGG-1-positive puncta were observed in the BC12921 and DA2123 worms, respectively, indicating that CRSE-induced autophagic flux in both cells and C. elegans models.
Figure 4|Citri Reticulatae Semen extract (CRSE) ameliorates amyloid-beta (Aβ)- and tau-induced in vivo pathologies in Caenorhabditis elegans.
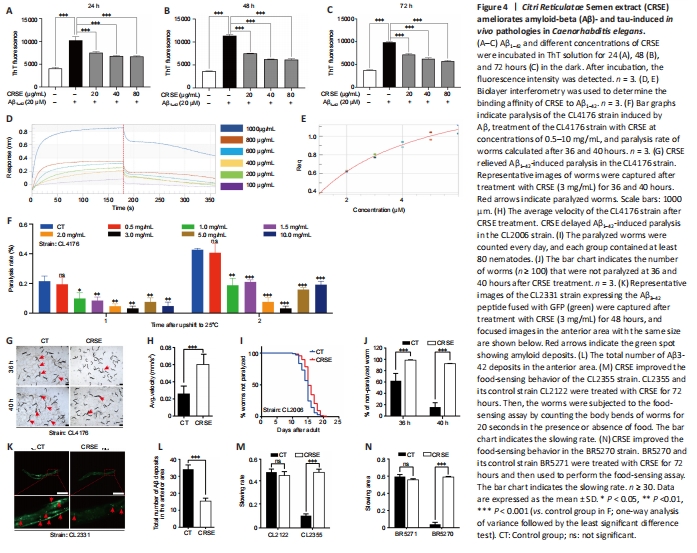
ThT is a benzothiazole stain that specifically binds to aggregated fibrils of Aβ and emits intense fluorescence at a specific wavelength of 480 nm, which is widely adopted for the experimental characterization of Aβ aggregation (LeVine, 1993). As shown in Figure 4A–C, CRSE inhibited the in vitro formation of Aβ1–42 fibrils from 24 to 72 hours of co-incubation with Aβ1–42. To validate the binding affinity of CRSE to Aβ1–42, the BLI assay which measures the biomolecular interaction between CRSE and Aβ was performed. As shown in Figure 4D and E, there was a dose-dependent increase in the optical thickness (nm) of the sensor layer, suggesting the direct binding of CRSE components to Aβ1–42. Furthermore, two strains of transgenic C. elegans in their body wall muscle cells, CL4176 and CL2006, in which human Aβ1–42 was temperature-sensitive and constitutively expressed, respectively, were used to investigate the effects of CRSE on Aβ-induced paralysis. Figure 4F shows that CRSE treatment significantly delayed the progress of paralysis in CL4176 worms, as indicated by the higher rate of unparalyzed worms both at 36 and 40 hours after CRSE treatments. Similar results were obtained in the CL2006 strain, in which the average paralysis time of CRSE treatment was delayed by 9% compared to the untreated group (Figure 4G–J). CL2331 strain containing temperature-sensitive GFP-linked human Aβ3–42 peptide in its body wall muscle cells was utilized to visualize Αβ aggregates. As shown in Figure 4K–N, CRSE treatment conferred a significant reduction in Αβ deposits in the anterior area of worms. CL2355 and BR5270 strains, in which human Aβ1–42 peptide and proaggregant F3ΔK280 tau protein fragment were ectopically overexpressed in the pan-neuronal cells of worms, respectively, were also adopted for further analysis. As expected, CRSE treatment significantly attenuated the food-sensing deficits in both CL2355 and BR5270 worms by increasing the slowing rate, and there was no significant difference in the slowing rate upon CRSE treatment in the CL2122 (with no Aβ production) and BR5271 (anti-Aβ aggregation) control worms. Taken together, these results suggested that CRSE ameliorated Aβ- or tau-induced pathologies in C. elegans.
Figure 5|Citri Reticulatae Semen extract (CRSE)-mediated autophagy activation delays amyloid-beta (Aβ)- and tau-induced pathologies in Caenorhabditis elegans.
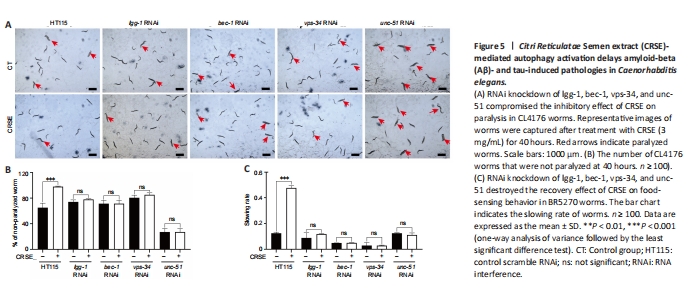
To determine whether CRSE exhibited a neuroprotective effect in the C. elegans AD model via autophagy induction, 4 key autophagy-related genes, lgg-1, bec-1, vps-34, and unc-51, were knocked down in both the CL4176 and BR5270 strains using RNAi technology, and the neuroprotective effects of CRSE were then evaluated. A paralysis assay revealed a significant increase in the percentage of unparalyzed worms in CL4176 C. elegans upon CRSE treatment, while knockdown of the key autophagy genes lgg-1, bec-1, vps-34, and unc-51 reduced this protective effect (Figure 5A), suggesting the dependence of the decelerated paralysis rates on autophagy activation. Meanwhile, a food-sensing response assay also showed that knockdown of the lgg-1, bec-1, vps-34, or unc-51 gene by RNAi successfully abolished the recovery of food-sensing deficits in BR5270 animals upon CRSE treatment (Figure 5B and C). Collectively, CRSE delayed Aβ- and tau-induced pathological phenotypes in an autophagy activation-dependent manner.
Figure 6|Citri Reticulatae Semen extract (CRSE) attenuates cognitive impairment, neuronal injury, and amyloid-beta (Aβ) burden in 3×Tg-AD mice.
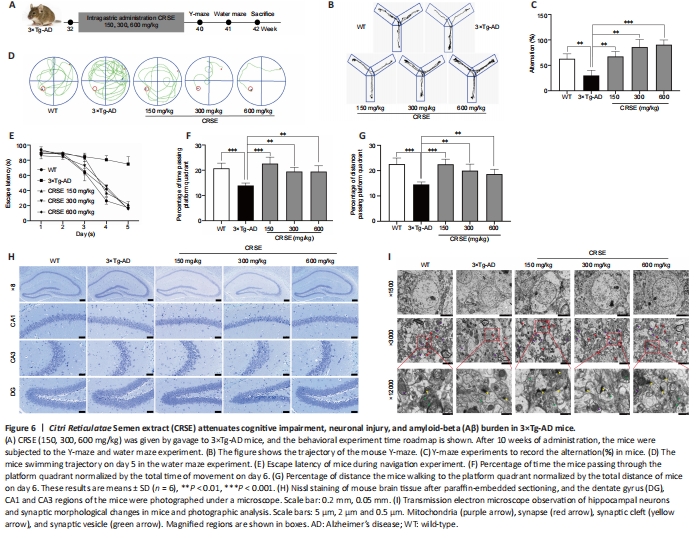
Figure 7|Citri Reticulatae Semen extract (CRSE) attenuates neuronal injury and amyloid-beta (Aβ) burden in 3×Tg-AD mice.
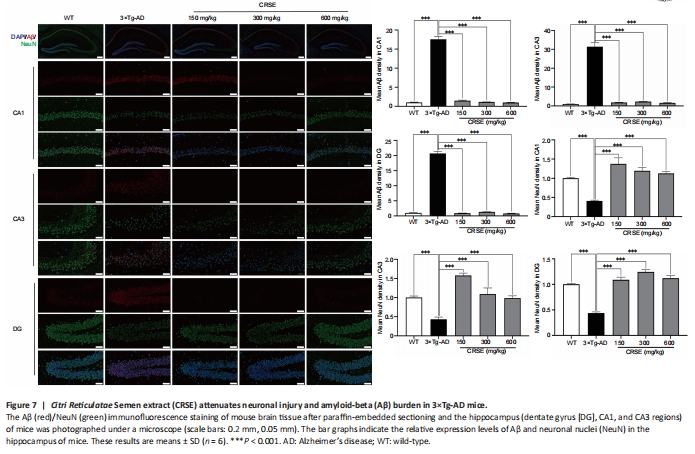
Figure 8|Citri Reticulatae Semen extract (CRSE) induces autophagy in 3×Tg-AD mice.
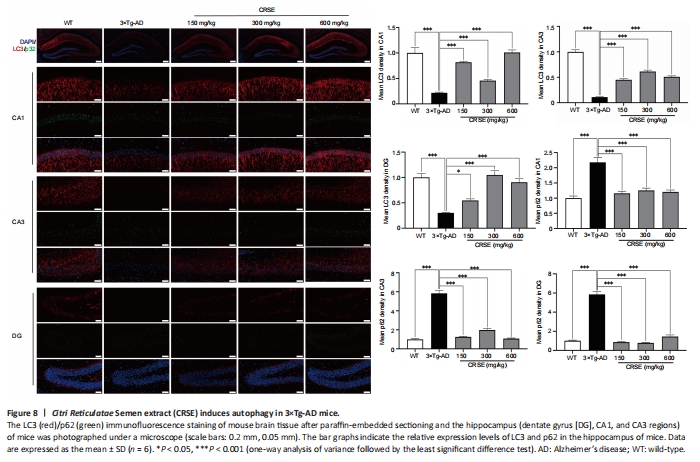
With the protective effect of CRSE on Aβ-induced cytotoxicity and C. elegans assays, the potential pharmacological effect of CRSE in Aβ-related learning and memory disorder was further validated in vivo. As shown by the increase in the percentage of alternation in the Y-maze experiment (Figure 6B and C), CRSE administration significantly improved the behavioral performance of the 3×Tg-AD mice compared to the vehicle-administered 3×Tg-AD group. In addition, the spatial working memory of CRSE (150 mg/kg)-treated 3×Tg-AD mice (CRSE) was recovered to a level comparable to the WT mice, as indicated by the number of platform crossings in the water maze test (Figure 6D–G). Pathologically, CRSE significantly increased the number of neurons and Nissl bodies (Figure 6H). The results of TEM showed that when compared to 3×Tg-AD control mice, the neuronal cell membrane was intact and continuous, the intracellular matrix was homogeneous, the number of mitochondria was increased with a reduction in swelling after CRSE intervention; the number of synaptic vesicles was increased and the synaptic gap was moderate (Figure 6I). While aggregation of neurotoxic Aβ was observed in the CA1, CA3, and DG regions of the 3×Tg-AD mice (Figure 7), neuronal loss in the hippocampal region was reduced after CRSE treatment. Consistently, the level of Aβ plaques in the hippocampus of the 3×Tg-AD mice was also decreased after CRSE treatment. Notably, Figure 8 shows that the level of LC3-II in the hippocampal region was significantly (P < 0.01) increased after CRSE intervention compared with that in the 3×Tg-AD control group. In addition, when compared with the 3×Tg-AD control group, the level of the autophagy substrate p62 was significantly (P < 0.01) decreased after CRSE treatment, indicating that CRSE enhanced autophagy with the concomitant amelioration of cognitive impairment and Aβ burden in the 3×Tg-AD mice.