周围神经损伤
-
Figure 3 | Effect of aligned fibers on guiding the growth of PC12 and DRG cells.
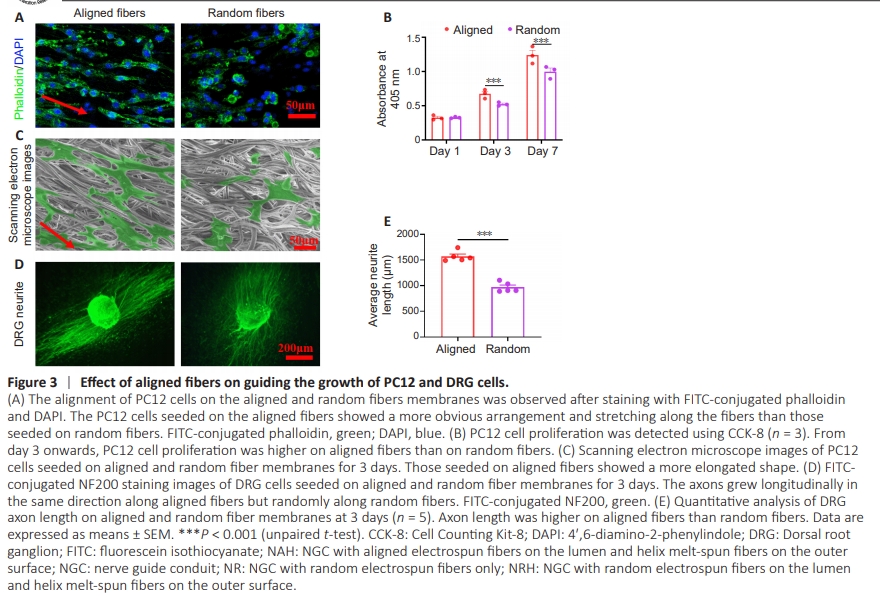
To investigate the effect of aligned and random fibers on nerve cell behavior, electrospun membranes with aligned and random electrospun fibers were seeded with PC12 cells and DRG cells, respectively. Phalloidin staining showed that PC12 cells aligned and extended along the direction of the aligned fibers; this did not occur along random fibers (Figure 3A). PC12 cell proliferation was significantly higher on the aligned fibrous membrane than the random fibrous membrane at 3 and 7 days (Figure 3B), indicating that aligned fibers promote nerve cell proliferation. Scanning electron microscope images confirmed the extension of PC12 cells on both aligned and random electrospun fibers (Figure 3C). DRG cells exhibited axons growing along the direction of the aligned fibers; however, axon growth was random on the random fibers (Figure 3D). Notably, DRG cell axon length was significantly longer on the aligned fibers than on the random fibers (Figure 3E). These results suggest that aligned fiber morphology on the NGC inner layer effectively guides neural and axon growth.
Figure 4 | Evaluation of resistance to kinking and compression properties in vivo.
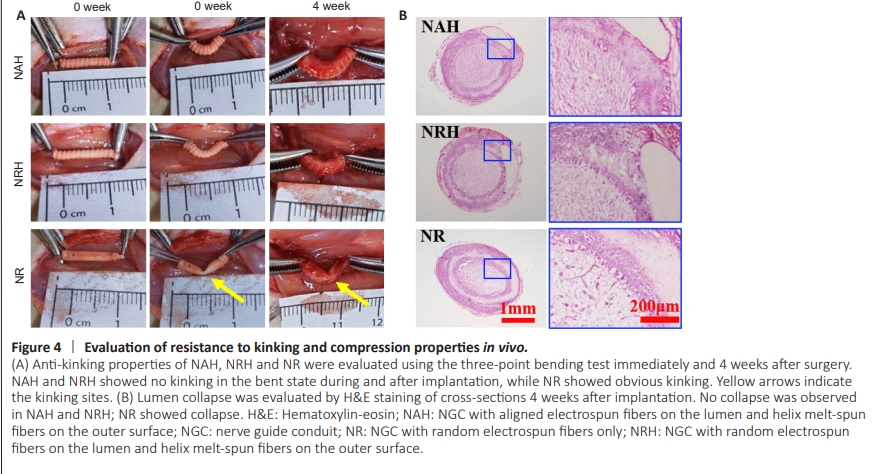
To evaluate NGC resistance to kinking and compression in vivo, each type was implanted into rat sciatic nerve defects. The NAH and NRH did not kink when subjected to a single pressure simulating surgically induced three-point bending. After removing the pressure, they returned to their tubular shape. Four weeks after implantation, the NAH and NRH maintained their tubular shape without any collapse. When subjected to unidirectional pressure, both NAH and NRH remained resistant to kinking and returned to their original shape after pressure removal; however, the NR still lacked the ability to resist kinking (Figure 4A). These results showed that NAH and NRH could maintain their tubular structure and exhibit the ability to resist pressure from muscles; in addition, they showed in vivo flexibility with joint bending. H&E staining confirmed that the NAH and NRH maintained their tubular shape 4 weeks after implantation (Figure 4B); however, the NR had already assumed an oval shape, probably because of pressure exerted by the surrounding muscle. Although the electrospun fiber layer is thinner in NAH and NRH than NR, no significant fibrotic scar tissue was observed in the NAH and NRH lumens. This suggests that NAH and NRH effectively prevent excessive infiltration of fibroblasts and formation of scar tissue. The in vivo anti-kinking and anti-compression properties of NAH and NRH, along with their ability to inhibit infiltration of fibroblasts and scar tissue, should promote nerve regeneration.
Figure 5 | Analysis of nerve regeneration in NGC after 4 weeks of implantation.
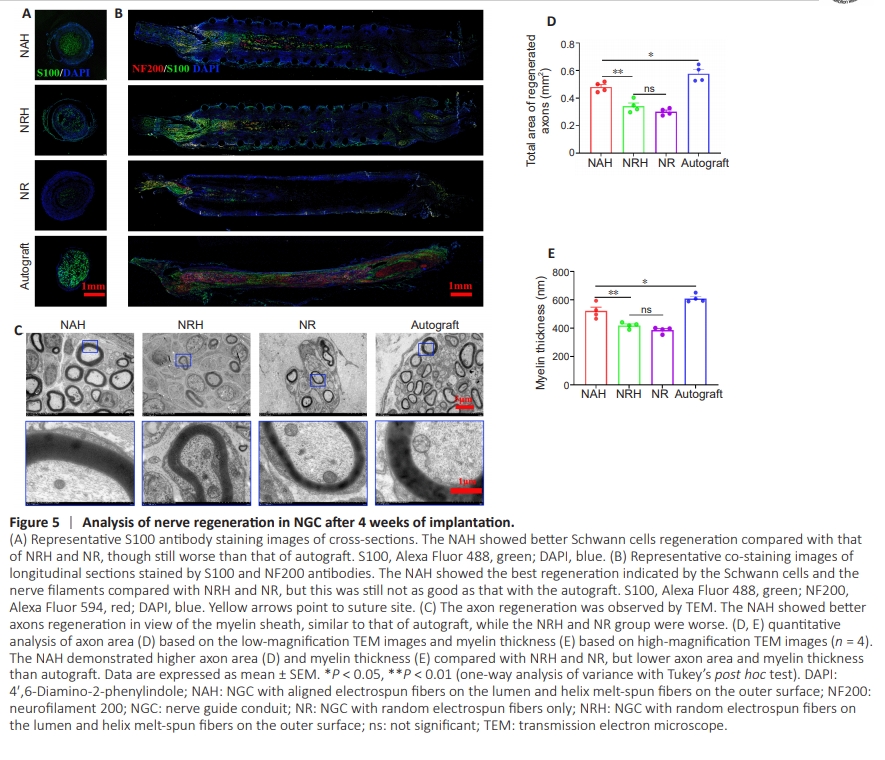
To evaluate the NGC ’s efficacy in promoting nerve regeneration, Schwann cells and neurofilaments were examined in explanted NGC samples using S100 and NF200 antibodies, respectively. The results showed that S100 expression was significantly higher in NAH than in NRHand NR (Figure 5A). When co-staining with NF200 and S100 antibodies was performed on longitudinal sections, we observed that Schwann cells within the NAH migrated distally along the nerve defect; in addition, nerve filament regeneration extended up to half the length of the defect (Figure 5B). These findings occurred to lesser degrees in NRH and NR. Furthermore, transmission electron microscopy (TEM) was performed to examine axon regeneration at a finer structural level (Figure 5C). The TEM images revealed that while the myelin sheath in NAH exhibited variation, with some axons appearing unmyelinated or hypomyelinated, quantitative analysis of axon area showed that mean axon area was greater in NAH (0.48 ± 0.01 mm2 ) than in NRH (0.34 ± 0.02 mm2 ) and NR (0.30 ± 0.01 mm2 ) (Figure 5D). Myelin sheath thickness was also significantly greater in NAH (521.50 ± 27.19 μm) than NRH (419.25 ± 10.76 μm) and NR (385.75 ± 10.78 μm) 4 weeks after implantation (Figure 5E). However, nerve regeneration was lower in NAH than in autografts, as indicated by NF200 and S100 antibody co-staining as well as TEM. This may be because the evaluation was conducted at only 4 weeks. Although not significant, both axon area and myelin sheath thickness were higher in NRH than in NR, indicating that both the aligned fibers and the flexibility of NAH play important roles in promoting axon regeneration. These results suggest that NAH promotes nerve regeneration through its structural design and topological cues in the lumen.