神经退行性病
-
Figure 2 | Validation of the expression levels of the hub genes in an MPTP-induced mouse model of PD.
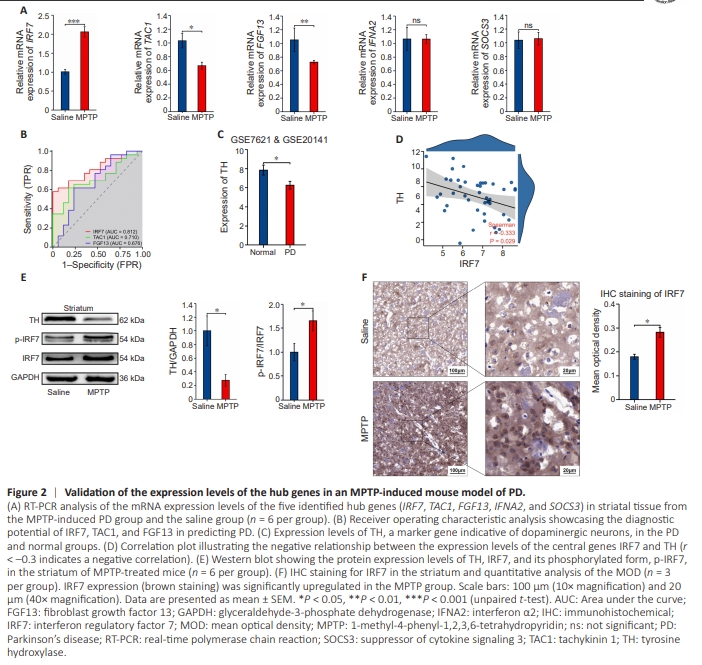
Next, we performed a preliminary literature review and chose to further investigate 5 of the 10 hub genes identified that have been less frequently mentioned or studied in the context of PD, specifically: TAC1, IRF7, FGF13, SOCS3, and IFNA2. We assessed the mRNA levels of these genes in PD mouse striatal tissue. Notably, IRF7 was expressed at significantly higher levels in the MPTP group than in the saline group. Furthermore, both TAC1 and FGF13 expression levels were notably reduced in the MPTP group compared with levels in the saline group. Conversely, SOCS3 and IFNA2 expression levels did not differ significantly between the MPTP and saline groups (Figure 2A). To evaluate the potential utility of IRF7, TAC1, and FGF13 as diagnostics markers for PD, we conducted an ROC analysis. The areas under the curve for IRF7 0.812, suggesting that would be highly accurate in predicting PD (Figure 2B). Expression levels of the marker gene TH, which is indicative of dopaminergic neurons (Hornykiewicz, 1998), were negatively correlated with IRF7 expression levels, as depicted in Figure 2D. This is consistent with the decreased TH expression observed in both patients with PD (Figure 2C) and mice with MPTP-induced PD (Figure 2E), suggesting irreversible damage to dopaminergic neurons. Further analysis by western blot showed significant upregulation of p-IRF7/IRF7 in the MPTPinduced PD mouse model (Figure 2E), suggesting that IRF7 may be actively involved in PD-associated inflammation. Additionally, immunohistochemical staining of the striatum showed a pronounced increase in the IRF7-positive area in the MPTP group compared with that in the saline group (Figure 2F).
Figure 3 | Analysis of IRF7 expression, immune cell infiltration, and mitochondrial function in a mouse model of PD and in BV2 cells.
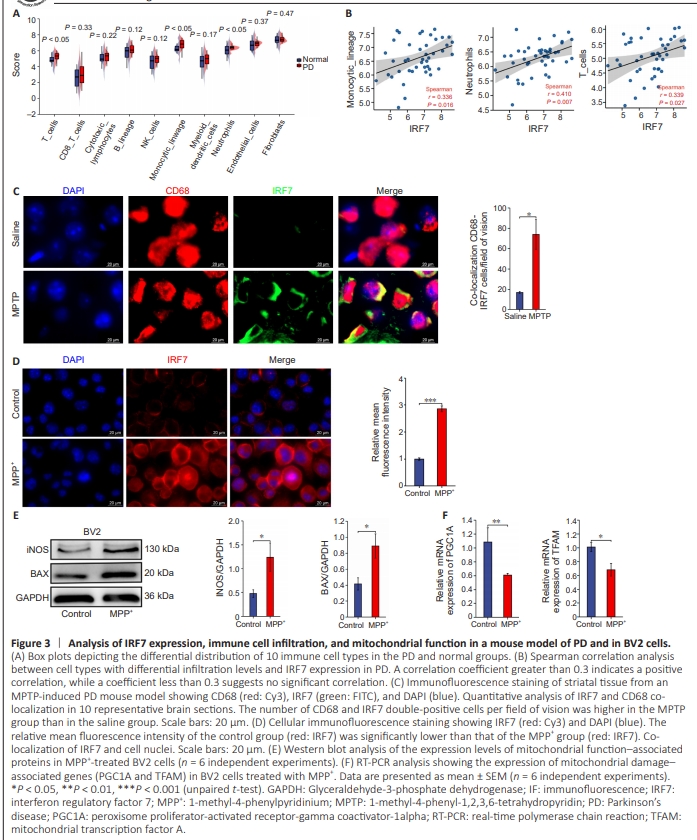
Next, we used the Microenvironment Cell Populations counter algorithm to analyze the immune activity of 10 types of immune cells in the PD and normal groups (Figure 3A). We observed notable differences in the numbers of infiltrating T cells, monocytes, and neutrophils between the PD and normal groups (Figure 3A). Further analysis showed a positive correlation between IRF7 expression and infiltration of T cells, monocytes, and neutrophils (Figure 3B). Notably, microglial cells are the primary monocytes involved in inflammation in PD (Kim and Joh, 2006). Thus, our findings suggest that IRF7 is associated with microglial cell infiltration in PD. To confirm this, we assessed IRF7 co-localization with microglial cells via immunofluorescence in MPTP-induced PD mice. Substantially higher levels of co-localization were detected in the striatal tissue of mice treated with MPTP compared with the findings in the saline group (Figure 3C), suggesting that activation of IRF7 expression promotes microglial infiltration in PD. 1-Methyl-4-phenylpyridinium modulates the expression of interferon regulatory factor 7 and markers of inflammation and mitochondrial dysfunction in BV2 cells To further elucidate the role of IRF7 in neuroinflammation associated with PD, we investigated IRF7 expression in BV2 cells treated with varying concentrations of MPP+ (0–50 μM). The CCK-8 assay results showed a significant reduction in cell viability upon treatment with 20 μM MPP+ (Additional Figure 2A). RT-PCR analysis confirmed a marked upregulation in IRF7 expression in cells treated with 20 or 50 μM MPP+ (Additional Figure 2B). As the MPP+ concentration increased, the expression levels of inflammatory cytokines TNF-α and IL-6 also increased (Additional Figure 2B). Furthermore, immunofluorescence analysis showed a significant increase in the relative average fluorescence intensity of cells treated with 20 μM MPP+ compared with that of untreated cells (Figure 3D). Additionally, western blot analysis demonstrated that compared with the control group, there was a notable upregulation of the mitochondrial dysfunction–associated proteins iNOS and BAX in the MPP+ -treated group (20 μM) (Figure 3E). Finally, RT-PCR analysis showed significant downregulation of PGC1A and TFAM in the MPP+ -treated group compared to the control group, which are associated with mitochondrial damage and mtDNA release, in the MPP+ - treated group (20 μM; Figure 3F).
Figure 4 | The cGAS-STING pathway plays a pivotal role in regulating IRF7 activation and nuclear translocation.
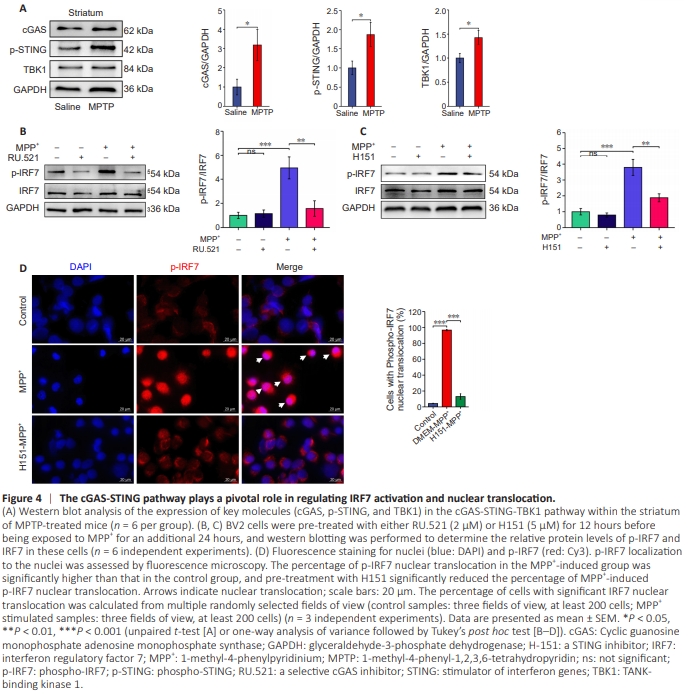
To investigate potential upstream regulators of IRF7, we used western blot analysis to detect the expression levels of cGAS-STING pathway components in striatal tissue. The results revealed significant upregulation of cGAS, p-STING, and tank-binding kinase 1 in the MPTP group compared with the saline group (Figure 4A). To determine the functional effect of these regulatory proteins, BV2 cells were pre-treated with either the cGAS inhibitor RU.521 or the STING inhibitor H151, followed by treatment with MPP+ . Western blot analysis showed a significant increase in p-IRF7 levels in the MPP+ - treated group, indicating IRF7 activation. This elevation was notably diminished by pre-treatment with either RU.521 or H151 (Figure 4B and C), suggesting that the cGAS-STING pathway plays a pivotal role in modulating IRF7 activation. Furthermore, immunofluorescence analysis demonstrated an evident increase in nuclear localization of p-IRF7 in the MPP+ -treated group, indicating that it relocated to the nucleus after activation. Notably, p-IRF7 nuclear translocation was considerably diminished following pre-treatment with H151 (Figure 4D), underscoring the impact of the STING pathway on IRF7nuclear translocation.