神经退行性病
-
Figure 1 | Several neurons in the CA1 and CA3 regions of the hippocampus of pR5 mice exhibit strong AT8 immunoreactivity and TAU missorting.
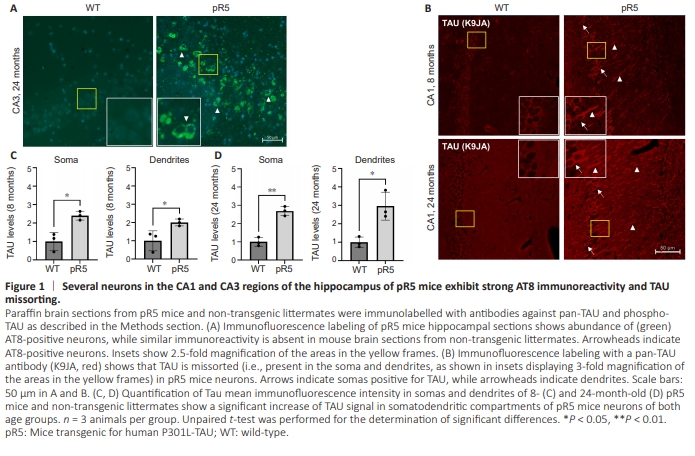
Microtubule stability is of key importance for proper neuronal function. We thus aimed to investigate the hallmarks and progress of TAU pathology in an in vivo model of tauopathy and its potential downstream effects on major PTMs of microtubules indicative of their stability and dynamics. We first stained brain sections of pR5 mice (transgenic for P301L-TAU) and age-matched non-transgenic littermates for TAU localization and phosphorylation. TAU was hyperphosphorylated (more than 20-fold increase intauopathy-typical AT8 phosphorylation, Figure 1A), and markedly missorted to the somatodendritic compartments of neurons in the hippocampi of pR5 mice as early as 8 months of age (Figure 1B–D). As aggregation of TAU in this mouse model only starts to occur at around 8 months of age (K?hler et al., 2013), this means that TAU missorting likely precedes the appearance of the first mature neurofibrillary tangles.
Figure 2 | Microtubules in neurons in the CA3 region of the hippocampus and subiculum of pR5 mice exhibit stability-decreasing changes in their essential post-translational modifications.
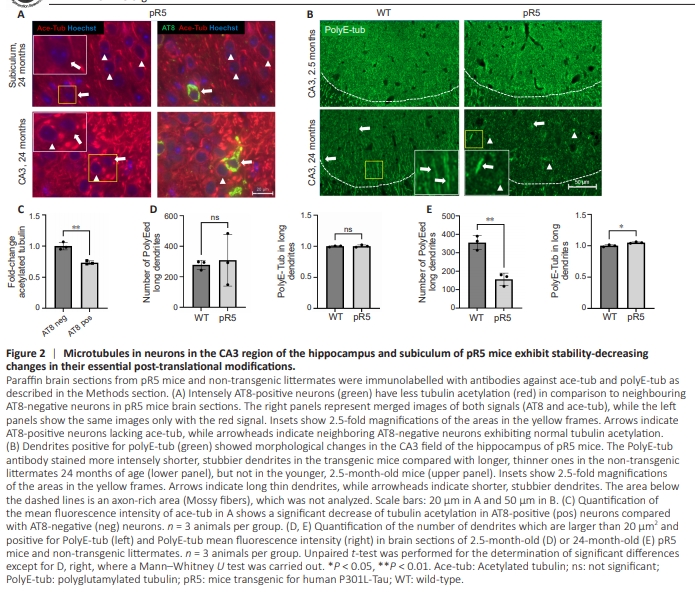
Next, we stained for acetylation and polyglutamylation of microtubules. Intensely AT8-positive neurons in 24-monthold pR5 mice exhibited a statistically significant decrease (~30%) of acetylated tubulin compared with neighboring, AT8-negative neurons (Figure 2A and C). Additionally, we observed morphological changes in the dendrites positive for polyglutamylated tubulin of 2-year-old pR5 mice, but the pattern of polyglutamylation did not show changes in the brains of the group of younger mice (Figure 2B and D). Dendrites were shorter and stubbier compared with longer and thinner dendrites abundant in the brains of nontransgenic littermates, and there was an obvious decrease (~45%) in the number of polyglutamylation-positive long dendrites (Figure 2B and E). In addition, the remaining long thin dendrites in 2-year-old pR5 mice showed a slight, but statistically significant increase in the fluorescence intensity of polyglutamylated tubulin (Figure 2B and E).
Figure 4 | 2N4R- and P301L-Tau exhibit similar sorting behavior when overexpressed in iNeurons.
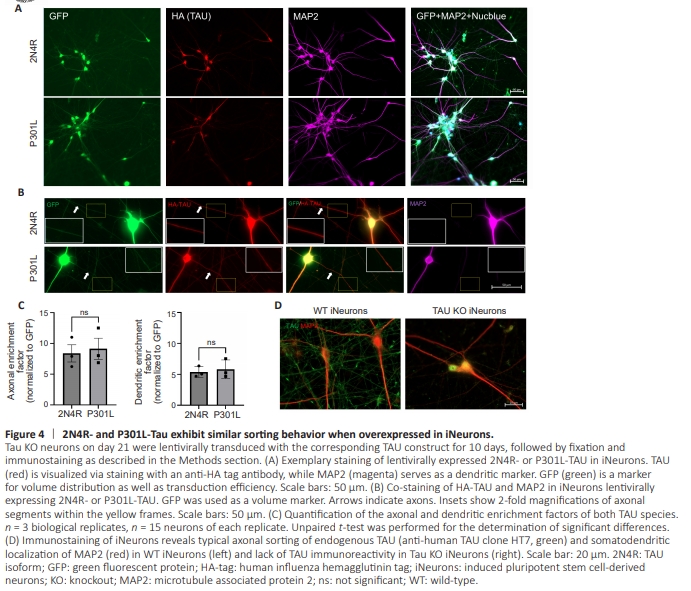
Next, we aimed to study P301L-TAU in neuronal cell culture, to gain an in-depth understanding of its axodendritic sorting and effect on microtubule PTMs. Primary neuronal cultures from the hippocampus of postnatal pR5 mice did not show specific human TAU immunolabelling using a human TAUspecific antibody in a significant number of cells (HT7, < 1/1000 cells, data not shown, while the antibody worked well in human neurons, Figure 4D). This is likely due to promoter incompatibility with immediate postnatal preparations of primary neurons. This renders primary neurons from the pR5 mice unsuitable as a cell model to study the consequences of human P301L-TAU expression. Therefore, the next step was to validate the previous findings regarding the effect of P301L-TAU on TAU sorting and phosphorylation, and on the PTMs of microtubules, in a human-relevant cell model. For this, we used human induced pluripotent stem cells-derived neurons (iNeurons). WTC11 cells with an additional transgene, Neurogenin2, were differentiated into excitatory cortical neurons with extremely high efficiency (> 90%), and within 3 weeks, via a differentiation protocol that provides minimal neuronal survival factors (N2, BDNF, NT3 in standard neuronal differentiation medium) but with the addition of doxycycline (Wang et al., 2017). In addition, our lab established MAPTKO iPSC lines, which can also be differentiated into the same neuronal subtype without effects on neuronal activity or properties (Bachmann et al., 2023). To introduce P301L-TAU into the cells, we cloned the coding sequence of the MAPT gene, carrying the mutation P301L associated with FTDP-17 and marked with an HA-tag, into a lentiviral vector (pUltra, which contains an eGFP marker, separated by a 2A peptide), on the basis of the longest TAU isoform, 2N4R-TAU. As a control, WT-MAPT cDNA coding for the longest TAU isoform 2N4R was also cloned in the same vector. iNeurons on day 21 after the start of differentiation were transduced with the corresponding lentiviral particles (Figure 4A), and the neurons were fixed 10 days after transduction. The sorting of 2N4R-TAU and P301L-TAU was investigated and compared as described previously (Bell et al., 2021). Unexpectedly, P301L-TAU did not show less efficient axonal targeting compared with wild-type 2N4R-TAU. Both 2N4Rand P301L-TAU favored localization to axons more than somatodendritic compartments, as indicated by their high axonal enrichment factors (AEF), which is defined as the axonto-soma ratio of TAU normalized to the axon-to-soma ratio of the randomly distributed volume/transduction marker GFP (AEF2N4R-TAU = 8.37 ± 2.42, AEFP301L-TAU = 9.12 ± 2.97) (Figure 4B and C). Dendritic enrichment factor (DEF) was also similar for both WT and mutant TAU, but less by around 40% than their AEFs, indicating an axonal preference for both expressed TAU, albeit with a prominent dendritic presence.
Figure 5 | 2N4R- and P301L-Tau exhibit different phosphorylation patterns when expressed in iNeurons.
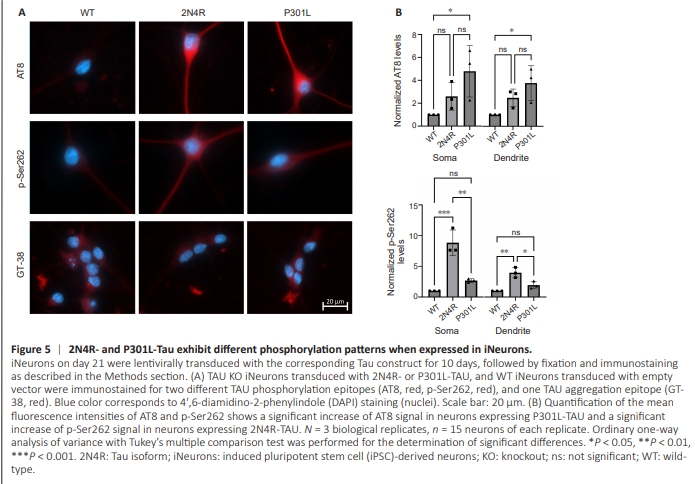
Similar to our finding in pR5 mice, overexpressed P301L-TAU showed a significant increase in AT8 reactivity compared with WT control. Interestingly, 2N4R-TAU also exhibited increased AT8 reactivity, albeit insignificant, compared with WT iNeurons (Figure 5). However, 2N4R-TAU showed a remarkable increase in the phosphorylation level on the Ser262 epitope (the first of KxGS motifs within the repeat domains), which was significantly higher than the level observed with P301LTAU (Figure 5). Furthermore, both WT- and P301L-TAU did not show any specific reactivity towards the conformationspecific GT-38 antibody, indicating the lack of any TAU aggregation following the expression of either of them (Figure 5). This means that the expression of either 2N4Ror P301L-TAU on TAU KO background results in increases in their phosphorylation on different epitopes compared with endogenous WT levels, but does not lead to TAU aggregation.
Figure 6 | Expression of 2N4R- or P301L-TAU alters some PTMs of microtubules in iNeurons.
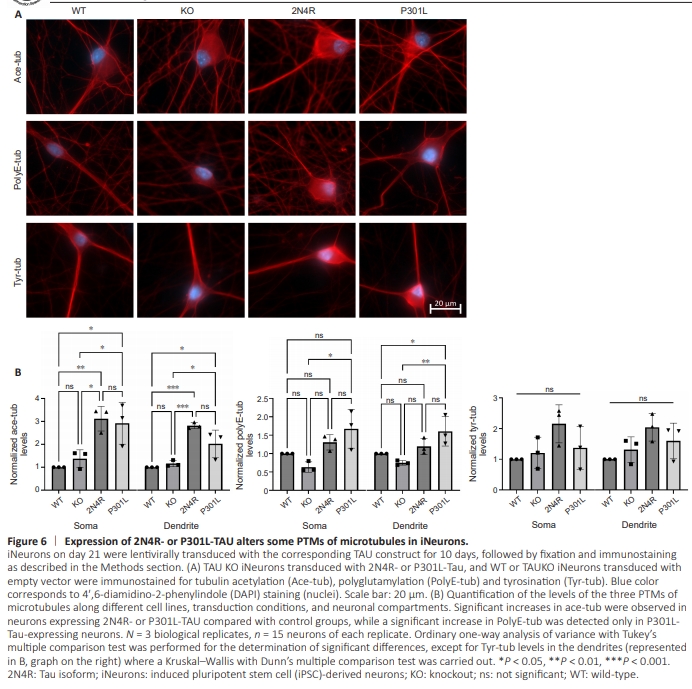
Next, the potential changes in the levels of different PTMs of microtubules following the overexpression of 2N4R- or P301L-TAU were investigated and compared in both somas and dendrites of transduced neurons. Acetylation levels were checked as a marker of stable microtubules. Expression of both TAU species significantly increased the levels of acetylated tubulin compared with control WT and TAU KO neurons transduced with empty vector (Figure 6). P301LTAU-transduced neurons showed slightly less acetylation than their WT-TAU-transduced counterparts did, more prominently in the dendrites. Interestingly, although insignificant, TAU KO neurons in general showed slightly increased levels of tubulin acetylation. When investigating the levels of tubulin polyglutamylation, P301L-TAU–transduced neurons exhibited increased polyglutamylation compared with WT and TAU KO control neurons. However, no significant changes in polyglutamylation were observed between P301L- and 2N4Rexpressing neurons (Figure 6). Finally, the expression of 2N4RTAU led to an insignificant increase in the levels of tubulin tyrosination. Despite not crossing the traditional threshold of significance, it was still noteworthy that P301L-expressing neurons had less tyrosinated tubulin compared with 2N4Rexpressing neurons (Figure 6).