神经退行性病
-
Figure 1 | Substrate bound amyloid-β peptides inhibit neurite growth in a dose-dependent manner.
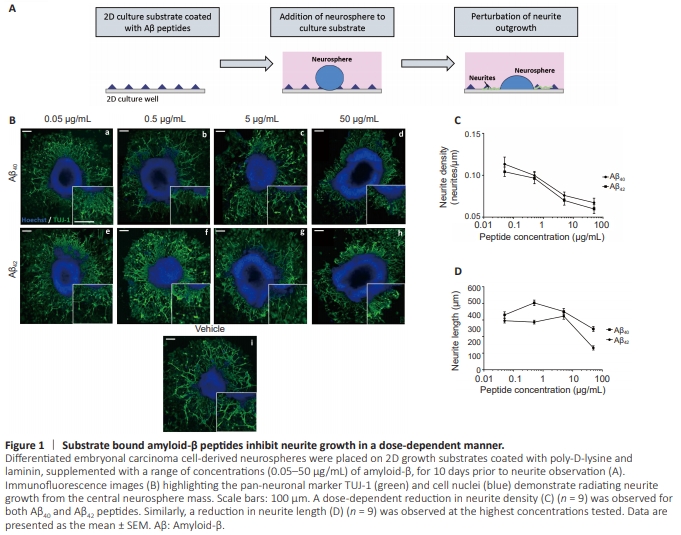
A previous study has demonstrated reduced neurite growth across Aβ-bound surfaces (Postuma et al., 2000). To test this in our EC cell-derived neuronal model system, we coated 2D culture substrates with a range of concentrations (0.05–50 μg/mL) of Aβ40 or Aβ42 and measured neurite outgrowth following 10 days (Figure 1A). TUJ-1 positive neurites radiated from the central neurosphere mass in all conditions (Figure 1B), with neurites visibly shorter and sparse at the highest concentration (50 μg/mL) for both peptides. Quantification of neurite density (Figure 1C) confirms a dosedependent decrease in neurite outgrowth with increasing concentrations of both Aβ40 and Aβ42, with fewer neurites present on Aβ42-coated substrates. Neurite length (Figure 1D) was particularly reduced at the highest concentration of peptide coating (50 μg/mL) and with Aβ42 coating as opposed to Aβ40.
Figure 2 | Exogenous Aβ reduces neurite outgrowth in physiologically relevant ratios.
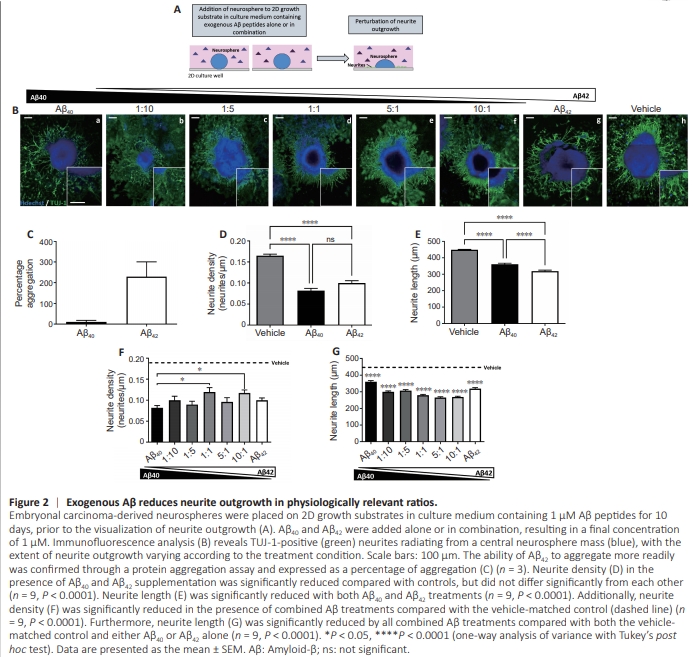
Although this data supports that of other studies described in the literature, in the brains of AD patients, neurites are not exposed to Aβ peptides in isolation but in combination, and the ratio of which is indicative of disease progression (Hansson et al., 2019). Therefore, we progressed to adding exogenous Aβ to the culture medium of neurospheres, either alone or in combination at a variety of ratios (Figure 2A). Similarly, neurite growth was identified (Figure 2B) as TUJ-1 positive neurites radiating from the central neurosphere mass. The enhanced capacity of Aβ42 to aggregate in this environment was confirmed through a protein aggregation assay (Figure 2C). Neurite density (Figure 2D) was significantly reduced both in the case of Aβ40 (P < 0.0001) and Aβ42 (P < 0.0001) in isolation compared with the vehicle control, but not significantly distinguishable from each other. Neurite length (Figure 2E) was also significantly reduced for both Aβ40 (P < 0.0001) and Aβ42 (P < 0.0001) treatments, but in this case, Aβ42 treatment resulted in significantly shorter neurites than Aβ40 treatment alone (P < 0.0001). When applied in combination, all Aβ conditions resulted in significantly (P < 0.01) reduced neurite density compared with vehicle-matched controls (Figure 2F), however, the extent of the reduction varied. Whereas neurite length (Figure 2G) was significantly reduced in all Aβ conditions compared with the vehicle control and was further significantly (P = 0.0001) reduced in all combination treatments compared with either Aβ40 or Aβ42 alone. An Aβ42/40 ratio of 1:10 is typical of a healthy brain (N?slund et al., 1994), whereas increasing Aβ42/40 ratios of 5:1 and 10:1 are indicative of an AD phenotype and resulted in the shortest neurites measured.
Figure 3 | Modulation of NgR signalling restores neurite outgrowth in the presence of inhibitory Aβ40.
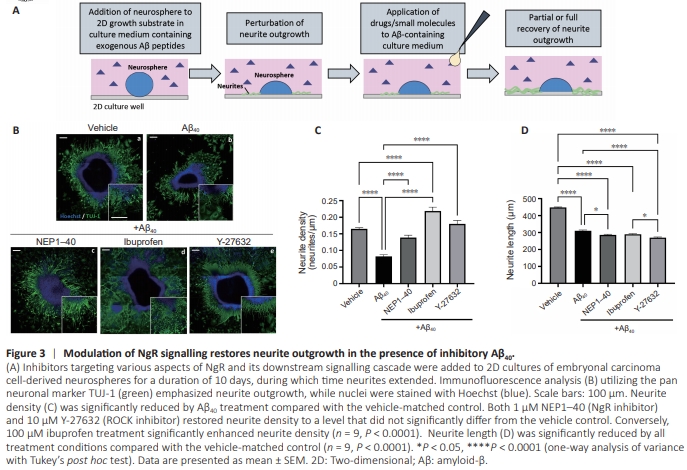
The glial scar is the inhibitory environment that arises postspinal cord injury (SCI) and has been widely studied. It involves the activation of receptors, including the Nogo receptor (NgR), by inhibitory molecules released from damaged neurons, resulting in the perturbation of actin dynamics via Rho A andROCK, ultimately leading to growth cone collapse and neurite retraction (Schwab, 2010). It has been speculated that Aβmediated neurite inhibition may share commonalities with this pathway through the interaction of Aβ with NgR (Park and Strittmatter, 2007). To investigate this in 2D culture, we added a combination of 1 μM Aβ40 with NgR pathway inhibitors to the culture medium of neurospheres during the 10-day neurite outgrowth period (Figure 3A). NEP1–40, a competitive inhibitor of the NgR, Y-27632, a ROCK inhibitor, and ibuprofen, which inhibits Rho A, were all used to elucidate the molecular mechanisms that underlie Aβ-mediated neurite inhibition. Immunofluorescence analysis of TUJ-1 and nuclei reveals significant neurite outgrowth from samples (Figure 3B). Neurite density measurements (Figure 3C) reveal significantly reduced neurite outgrowth due to Aβ treatment (P < 0.0001). Restoration of neurite growth to levels that did not significantly differ from the vehicle-matched control was observed through NEP1–40 and Y-27632 treatments, and enhancement of neurite growth that significantly surpassed that of the vehicle control was achieved by ibuprofen treatment (P < 0.0001). Neurite length (Figure 3D), however, was significantly reduced in all conditions compared with the vehicle-matched control (P < 0.0001).
Figure 4 | Modulation of Rho A/ ROCK signalling attenuates Aβ40- mediated neurite inhibition in 3D culture.
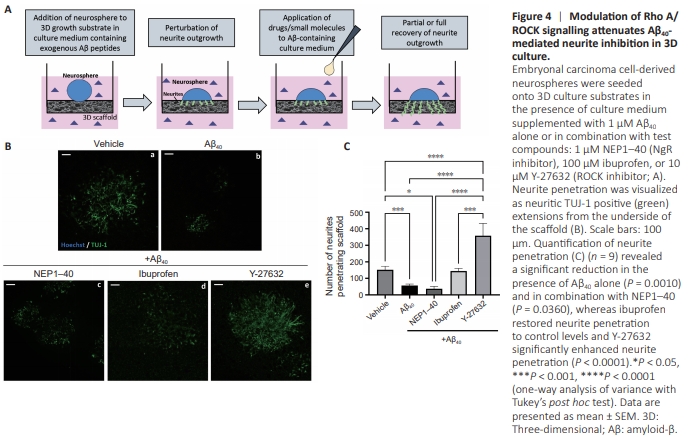
A 3D culture environment was also adopted to provide a more physiologically relevant geometry for developing neurites. This well-characterized model of neurite outgrowth has been applied in many previous studies, providing a robust, quantifiable assay (Clarke et al., 2017; Goncalves et al., 2023). Neurospheres were placed onto the 3D scaffold for the 10- day neurite outgrowth phase of culture, with the same combination of molecules added to the culture medium: Aβ alone or in combination with NEP1–40, ibuprofen, or Y-27632 (Figure 4A). Neurites were visualized as TUJ-1 positive protrusions visible from the underside of the scaffold (Figure 4B), having fully penetrated the material. Neurite penetration (Figure 4C) was significantly reduced in the presence of Aβ alone (P = 0.0010), confirming 2D findings. However, neurite penetration was not restored by the addition of NEP1–40 and was still significantly reduced compared with controls (P = 0.0366). Ibuprofen treatment restored neurite penetration to a level that did not significantly differ from the vehicle control, whereas Y-27632 enhanced neurite penetration to a level that surpassed that of the control (P < 0.0001).
Figure 5 | Neurite outgrowth from neurospheres that possess AD-associated mutation is reduced and rescuable through Rho A/ROCK modulation in 2D culture.
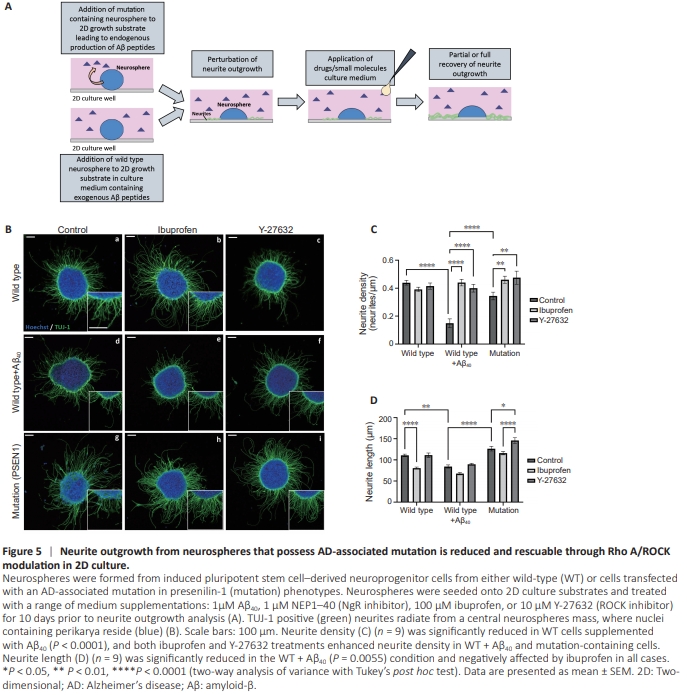
To further increase the complexity and physiological relevance of this assay system, we incorporated iPSC-derived neuroprogenitor cells that express an AD-associated mutation (PSEN1) and endogenously secrete an elevated Aβ42/40 ratio. The same method was applied, whereby neurospheres were seeded onto 2D growth surfaces in the presence of an array of small molecules or drugs and cultured for 10 days to allow for neurite outgrowth (Figure 5A). Neurite growth from wildtype (WT) cells with (Figure 5Bd–f) and without (Figure 5Ba– c) exogenous Aβ40 was observed, as well as in combination with ibuprofen (Figure 5Be) or Y-27632 (Figure 5Bf). Similarly, mutation cells were cultured without supplementation (Figure 5Bg) or supplemented with ibuprofen (Figure 5Bh) or Y-27632 (Figure 5Bi). Neurite density (Figure 5C) was significantly reduced in WT cells exposed to exogenous Aβ40 (P < 0.0001) and mutation cells. Ibuprofen (P = 0.0038) and Y-27632 (P = 0.0046) significantly enhanced neurite outgrowth to a level comparable to WT in the presence of exogenous Aβ40. Similarly, ibuprofen (P = 0.0038) and Y-27632 (P = 0.0046) treatment significantly enhanced neurite outgrowth from mutation cells to a similar level as WT. However, although neurite length (Figure 5D) was significantly reduced in WT with exogenous Aβ40 (P = 0.0055), there was no significant difference between mutation and WT cells. Ibuprofen reduced neurite length in all conditions, significantly in the case of WT (P < 0.0001). However, Y-27632 had little effect on neurite length in WT cells with or without exogenous Aβ40, but did significantly increase length in the case of mutation cells (P = 0.0377) compared with control cells.
Figure 6 | Neurite outgrowth in 3D is reduced from neurospheres that possess AD-associated mutation.
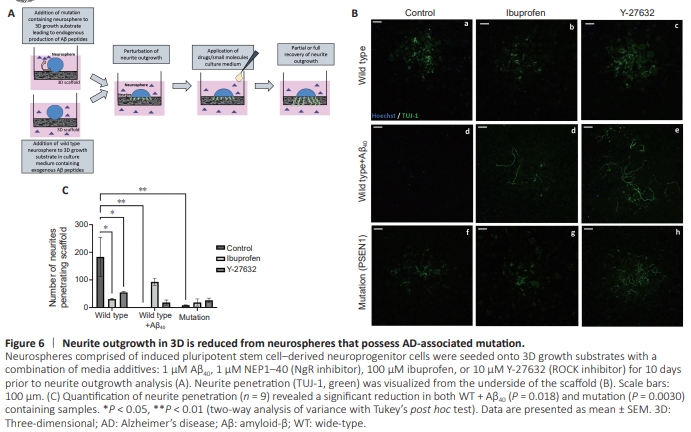
This study was then translated into a 3D environment more reminiscent of in vivo neurite development, by adding neurospheres derived from WT or mutation phenotype iPSCs onto a 3D culture substrate in the presence or absence of test compounds (Figure 6A). Neurite penetration from the underside of the scaffold was visualized as TUJ-1-positive neurites (Figure 6B). Quantification (Figure 6C) revealed significantly reduced neurite penetration in WT cells supplemented with Aβ40 (P = 0.0018) and mutation-containing cells (P = 0.0030). Ibuprofen (P = 0.0101) and Y-27632 (P = 0.0404) significantly reduced neurite penetration in WT cells, but increased neurite penetration to a small degree in cells supplemented with Aβ40 and mutation samples compared with respective controls.