神经退行性病
-
Figure 4 | The effects of DDQ treatment on immunoreactivity of MAP2 and neurite outgrowth in HT22 cells.
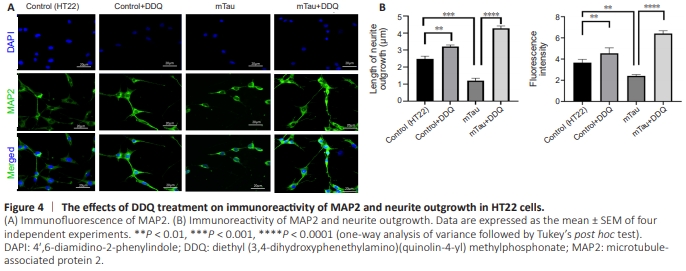
Immunofluorescence staining was used to validate MAP2, β-tubulin, synaptophysin, VAChT, and PGC1α and to examine neurite outgrowth and marker fluorescence in DDQ-treated HT22 neuronal cultures to determine the influence DDQ on neurite properties and neuronal growth (Figures 4–8) in four groups of HT22 cells. Significantly increased neurite length, neurite outgrowth (P = 0.0012), and immunoreactivity of MAP2 (P = 0.0045) were found in DDQ-treated HT22 cells compared with Control (HT22) cells (Figure 4B). Significantly decreased neurite length, neurite outgrowth (P = 0.0009), and immunoreactivity ofMAP2 (P = 0.0080) were found in mTau HT22 cells compared with control HT22 cells (Figure 4B). Significantly increased neurite length (P = 0.0001) and immunoreactivity of MAP2 (P = 0.0001) were found in DDQ + mTau cells compared with mHT22 cells (Figure 4B).
Figure 5 | The effects of DDQ treatment on immunoreactivity of β-tubulin and neurite outgrowth in HT22 cells.
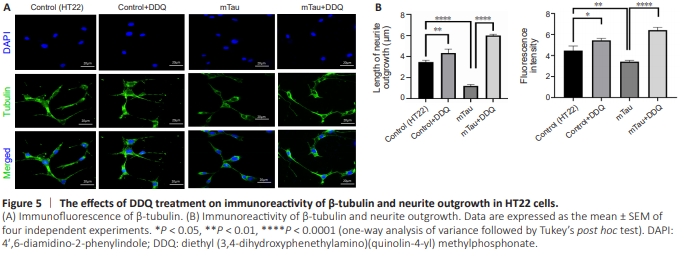
Significantly increased neurite length, neurite outgrowth (P = 0.0064), and immunoreactivity of β-tubulin (P = 0.0116) were found in DDQ-treated HT22 cells compared with Control (HT22) cells (Figure 5B). Significantly decreased neurite length, neurite outgrowth (P = 0.0003), and immunoreactivities of β-tubulin (P = 0.0001) were found in mTau HT22 cells compared with control HT22 cells (Figure 5B). Significantly increased neurite length (P = 0.0001) and immunoreactivity of β-tubulin (P = 0.0001) were found in DDQ + mTau cells compared with mTau (cDNA transfected mHT22 cells) (Figure 5B).
Figure 6 | The effects of DDQ treatment on immunoreactivity of synaptophysin and neurite outgrowth in HT22 cells.
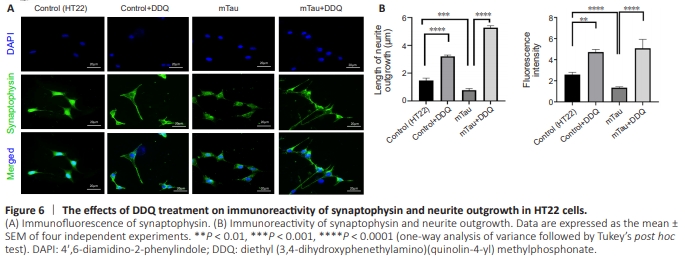
Significantly increased neurite length increased neurite outgrowth (P = 0.0001), and immunoreactivity of synaptophysin (P = 0.0019) were found in DDQ-treated HT22 cells compared with Control (HT22) cells (Figure 6B). Significantly decreased neurite length, neurite outgrowth (P = 0.0009), and immunoreactivity of synaptophysin (P = 0.0001) were found in mTau HT22 cells compared with control HT22 cells (Figure 6B). Significantly increased neurite length (P = 0.0001) and immunoreactivity of synaptophysin (P = 0.0001) were found in DDQ + mTau HT22 cells compared with mHT22 cells (Figure 6B).
Figure 7 | The effects of DDQ treatment on immunoreactivity of PGC1α and neurite outgrowth in HT22 cells.
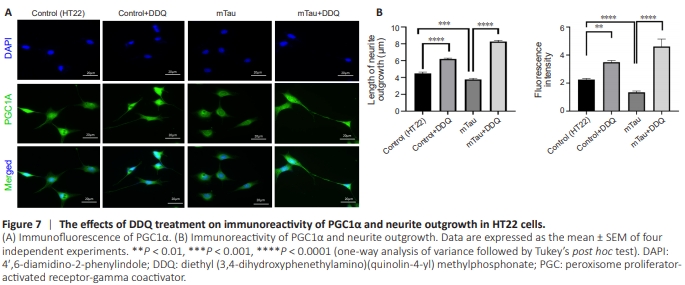
Significantly increased neurite length (P = 0.0001) and immunoreactivity of PGC1α (P = 0.0029) were found in DDQtreated HT22 cells compared with Control (HT22) cells (Figure 7B). Significantly decreased neurite length (P = 0.0023) and immunoreactivity of PGC1α (P = 0.0001) were found in mTau HT22 cells compared with control HT22 cells (Figure 7B). Significantly increased neurite length and (P = 0.0001) and immunoreactivity of PGC1α (P = 0.0001) were found in DDQ + mTau cells compared with mHT22 cells (Figure 7B).
Figure 8 | The effects of DDQ treatment on immunoreactivity of VAChT and neurite outgrowth in HT22 cells.
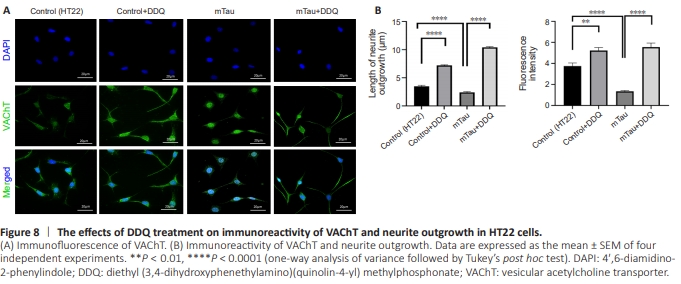
Significantly increased neurite length (P = 0.0001) and immunoreactivity of VAChT (P = 0.0011) were found in DDQtreated HT22 cells compared with Control (HT22) cells (Figure 8B). Significantly decreased neurite length (P = 0.0003) and immunoreactivity of VAChT (P = 0.0001) were found in mTau HT22 cells compared with control HT22 cells (Figure 8B). Significantly increased neurite length (P = 0.0001) and immunoreactivity of VAChT (P = 0.0001) were found in DDQ + mTau cells compared with mHT22 cells (Figure 8B).
Figure 9 | Comparison of neurite outgrowth between undifferentiated and differentiated HT22 cells.
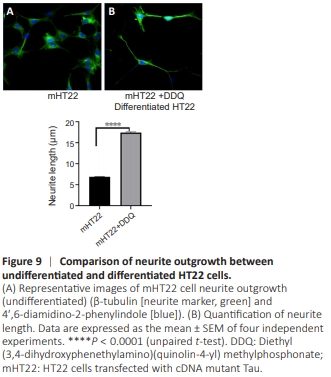
These findings sug gested that neurite length and immunoreactivity of MAP2, β-tubulin, PGC1α, and VAChT relative to the control HT22 cells and DDQ treatment show the optimal neuronal growth and immunoreactivity when treated with DDQ in control HT22 cells and in the presence of mTau (mTau cDNA transfected HT22 cells) neuronal toxicity conditions. HT22 cells presented important neuronal growth properties along with existing antioxidant and anti-aging properties after DDQ treatment. These findings showed that the differentiation of HT22 cells enhanced neurite outgrowth in the presence of mTau via β-tubulin marker compared with mTau cells alone (Figure 9).