周围神经损伤
-
Figure 1 | Immunohistochemistry analysis of KIF4A expression in the uninjured spinal cord.
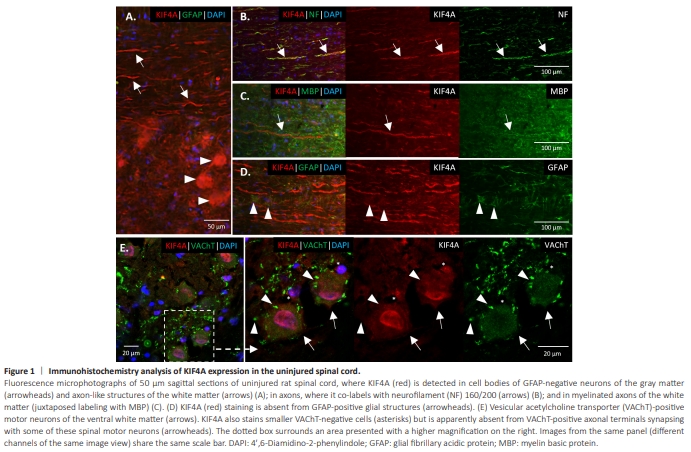
The lack of studies on KIF4A in adult nervous tissue (and even reports of its absence), together with its intriguing increased expression in animals with improved recovery from SCI, drove us to characterize KIF4A expression in adult CNS and PNS neural tissue, particularly in cells and structures relevant for PNS regeneration upon injury. Following validation of an anti-KIF4A antibody for rat and human tissue (Additional Figures 1 and 2), a first brief immunohistochemistry analysis of KIF4A distribution in the rat adult spinal cord was performed. Given that the KIF4A signal was present in both gray and white matter of the rat’s uninjured spinal cord (Figure 1). In the ventral gray matter, the KIF4A signal was observed in glial fibrillary acidic protein (GFAP)- negative large cell bodies (Figure 1A, arrowheads), further confirmed to be motor neurons. In the white matter, double labeling revealed an overlap between the axonal neurofilament (NF) marker and KIF4A, confirming the neuronal distribution and abundant axonal expression of this protein (Figure 1B). Moreover, KIF4A appeared to be present in CNS myelinated axons, as KIF4A-positive axons showed to be enclosed in structures positive for myelin basic protein (MBP) (Figure 1C). Co-labeling with the glial marker GFAP in the white matter revealed no evident KIF4A/GFAP overlap in the spinal cord (Figure 1D). At the ventral spinal cord, double staining of KIF4A and the cholinergic transporter vesicular acetylcholine transporter (VAChT) confirmed KIF4A expression by motor neurons (Figure 1E, arrows), where it exhibits a cytoplasmic distribution and a nuclear enrichment in various of these neurons. KIF4A staining was also observed in the nucleus of VAChTnegative smaller cells (Figure 1E, asterisks), in VAChT-positive fibers of the ventral white matter and in various cells of the dorsal horn (known to be highly enriched in interneurons) (Additional Figure 3). Interestingly, KIF4A did not visibly co-stained with large VAChT-positive axonal terminals synapsing with various spinal motor neurons (Figure 1E, arrowheads), suggesting that KIF4A is not expressed or expressed at very low levels in some brain and brainstem cholinergic neurons that directly synapse with lower motor neurons. Following SCI, KIF4A signal at the spinal cord is still evident, as well as its co-labeling with the neuronal marker NF; likewise, KIF4A labeling of larger neuronal cell bodies, particularly at their nuclei, persists in the gray matter in the spinal cord after the lesion of a downstream peripheral nerve (Additional Figure 4).
Figure 2 | KIF4A expression in cross sections of uninjured rat DRG.
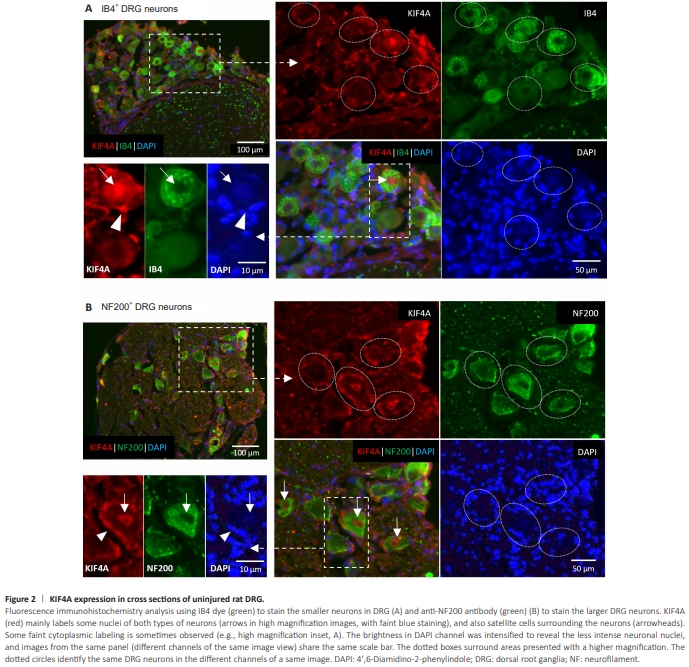
Immunohistochemistry analyses of adult rat DRG cross-sections demonstrated KIF4A expression in the soma of mature DRG neurons (Figure 2). KIF4A is expressed by different DRG neuronal subtypes: it is visible in large and mediumsized NF200-positive DRG neurons, which mainly represent myelinated touch and proprioceptive neurons, and also in small-diameter IB4-positive neurons, potentially unmyelinated neurons relaying nociception. Some IB4-negative small neurons, presumably peptidergic (CGRP-positive) nociceptive neurons, are also KIF4A-positive. Subcellularly, KIF4A is found in the cytoplasm of both IB4- and NF200-positive neurons, but also in the nuclei of some of these DRG neurons (Figure 2, arrows). In addition, KIF4A appears to be in contact zones between neurons and surrounding cells, most likely satellite cells (Figure 2, arrowheads), and may also be expressed by these glial cells that present an envelope-like distribution due to their typical morphology with extended thin processes around the neuronal bodies (Wang et al., 2019; Maniglier et al., 2022).
Figure 3 | KIF4A protein expression in rat uninjured peripheral nerves.
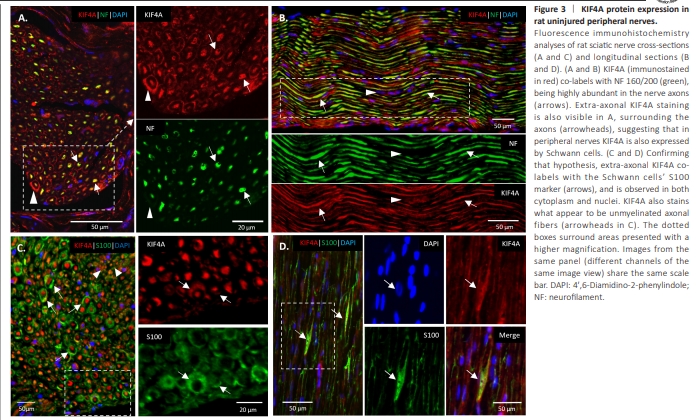
In an intact rat sciatic nerve, KIF4A double labeled with the NF axonal marker in a high number of tubular structures (Figure 3A and B, arrows), confirming KIF4A high abundance in the nerve’s axons. The staining clearly shows a prevalent KIF4A neuronal localization in the uninjured sciatic nerve. Nevertheless, a fainter KIF4A signal was observed in NF-negative neural cells surrounding the axons, whose nuclei can be seen on longitudinal sections (Figure 3A and B, arrowheads). KIF4A/S100 double labeling confirmed these as Schwann cells (Figure 3C and D, arrows), with KIF4A localizing at their cytoplasm, in some of their nuclei and plasma membrane.
Figure 4 | KIF4A protein expression in human peripheral nerves.
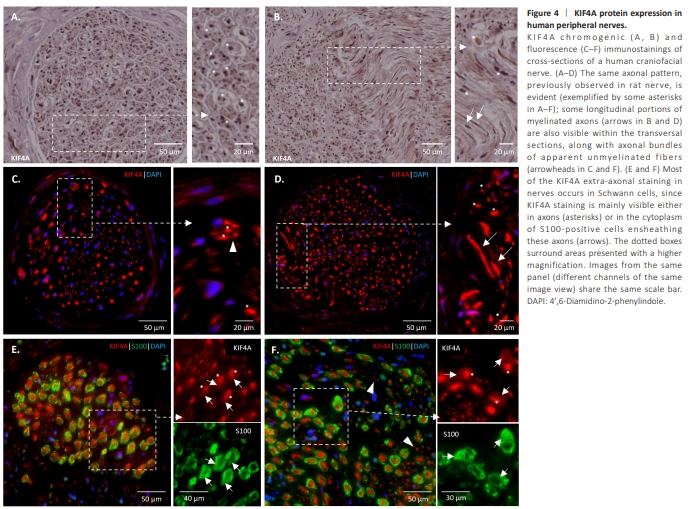
The same distribution of KIF4A was visible in an intact adult human nerve (Figure 4). A highly abundant axonal expression is clear, as shown by positive dots at the core of cross-sectioned nerve fibers (asterisks in Figure 4), or as tubular structures in some longitudinal areas of the nerve section (arrows in Figure 4B and D). Of note, KIF4A is not only present in myelinated axons but also in what appear to be unmyelinated ones (arrowheads in Figure 4C and F). In human nerves, as described above for rat nerves, KIF4A extra-neuronal staining is mainly observed in S100-positive Schwann cells, which also present KIF4A at their cytoplasm, although with fainter staining than in axons (Figure 4E and F, arrows). KIF4A is also expressed by a few endoneurial fibroblast-like (Richard et al., 2014) and endothelial cells of rat and human nerves, as shown by the double labeling of KIF4A and CD34, a marker for endothelial and hematopoietic stem/progenitor cells (Karaman et al., 2019), and of KIF4A and anti-nerve/glial antigen 2 (NG2) a perineurial and fibroblast-like cells marker (Chelyshev et al., 2022; arrows in Additional Figure 5).
Figure 5 | KIF4A expression in DRG neurons following peripheral nerve injury.
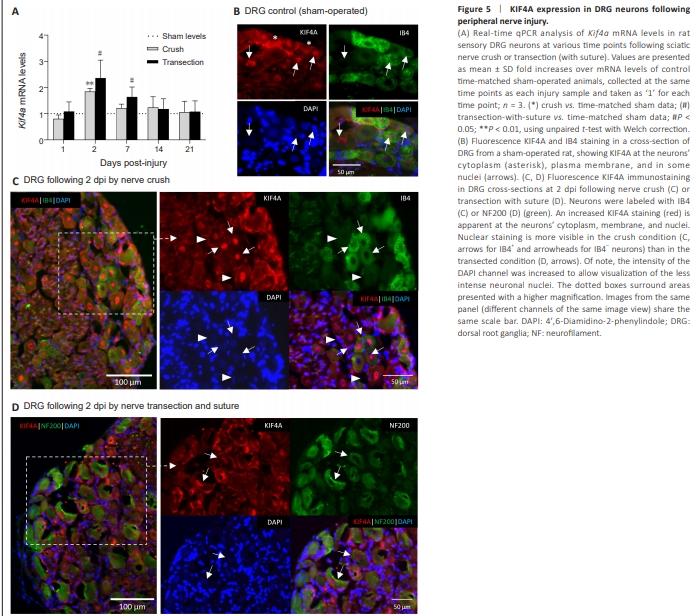
Kif4a mRNA levels were analyzed by real-time qPCR in the lesioned DRGs at various days post-injury (dpi). The resulting data revealed a lesion-induced regulation of Kif4a mRNA (Figure 5A). Upon sciatic nerve crush, Kif4a exhibits maximal up-regulation at 2 dpi (~1.8-fold, P < 0.01) and slowly declines back to basal levels (sham condition) within 2–3 weeks. After transection, a similar expression profile was observed, with a slightly stronger initial up-regulation (~2.4-fold, P < 0.05) at 2 dpi and a subsequent slower decline to basal levels. Parallel immunohistochemical analysis of rat DRG sections upon 2 dpi supported an apparent increase of KIF4A protein signal in the DRG cells of the injured nerves (Figure 5C and D) compared with KIF4A staining in DRG control sections (Figures 2 and 5B). A similar pattern of KIF4A protein distribution was observed in injured neurons after either crush or transection lesions, with high KIF4A signal being observed at the neuronal cytoplasm and particularly at their plasma membranes (and potentially in the satellite cells). Higher KIF4A nuclear staining was also visible, although more homogeneously in crushed neurons [either IB4-positive (arrows) or negative (arrowheads)] (Figure 5C), than in transected and sutured neurons (Figure 5D, arrows).
Figure 6 | KIF4A mRNA and protein expression in the distal stump of an injured nerve.
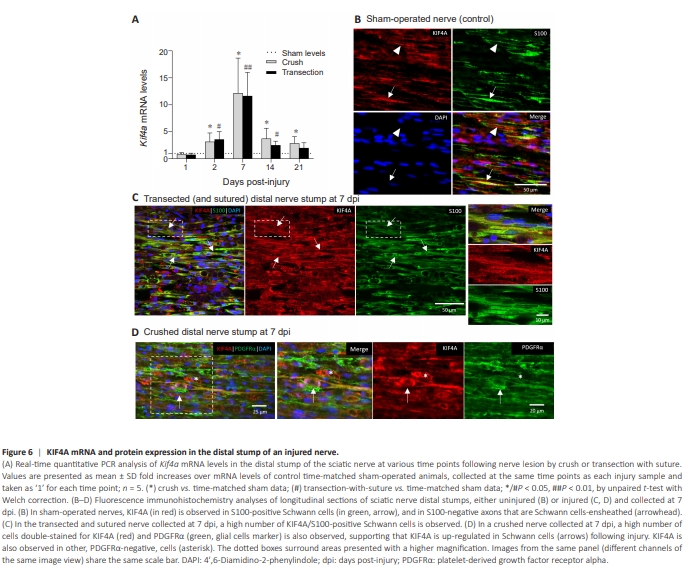
Alterations in Kif4a expression at the distal nerve stump were also monitored, since this is a region of the nerve tissue that is particularly subjected to large alterations after traumatic injury. A major injury-induced Kif4a up-regulation could be observed in the distal stumps of both crushed and transected sciatic nerves (Figure 6A). Kif4a mRNA was significantly increased already at 2 dpi, reaching a maximum at 7 dpi in both lesion paradigms (~11.7–12.1-fold increase over levels in sham-operated animals; P < 0.01 for transection and P < 0.05 for crush conditions; Figure 6A). In both lesion paradigms, Kif4a mRNA levels subsequently declined over time to ones closer to the basal sham levels, although in the crush condition and at 21 dpi they significantly remain above those of sham-operated animals. Immunohistochemistry analysis of the distal stumps showed absence or near absence of neurofilament labeling in distal sections of a transected and sutured nerve, at either 7 or 21 dpi, revealing complete axon degeneration as expected (Additional Figure 6A and B). Indeed, axonal degeneration and Schwann cells proliferation and reprogramming into a repair phenotype can occur in both lesion paradigms, while regenerative axonal ingrowth only occurs in the pre-crushed distal stump and is absent in the ligated transected distal stump (Dun and Parkinson, 2018; Jessen and Mirsky, 2019). Given this, the stained tissue in sagittal sections of a crushed distal nerve fragment, collected at 7 dpi in an area adjacent to the lesion, appeared relatively normal (uninjured) (Figure 6D versus Figure 6B). Consistently, increased mRNA levels of the POU transcription factor Oct6, a marker of promyelinating Schwann cells (Liu et al., 2015; Zhang et al., 2023), were observed in the crushed distal stump at 7–21 dpi, indicating induction of axon remyelination in this regenerative condition. Contrary, Oct6 mRNA levels remained very low in the transected and ligated fragment (Additional Figure 6C). These data suggest that the lesion-induced Kif4a upregulation observed in both crushed and transected distal nerve stumps (Figure 6A), occurs in non-neuronal cells, such as distal Schwann cells, independently of the regenerative myelination process. Indeed, double staining of the injured distal fragment for KIF4A and S100 confirmed that the KIF4A protein signal was clearly detected in S100- positive Schwann cells (arrows in Figure 6C). Complementary staining with the glial (and endothelial) marker PDGFRα also revealed double labeling of PDGFRα and KIF4A signals (Figure 6D). Although less frequent, some KIF4A-positive but PDGFRα-negative cells were also observed, which likely correspond to fibroblasts or other endoneurial cells (Figure 6D, asterisk).
Figure 7 | KIF4A expression in rat regenerating sciatic nerve fascicles, upon nerve transection with conduit implantation.
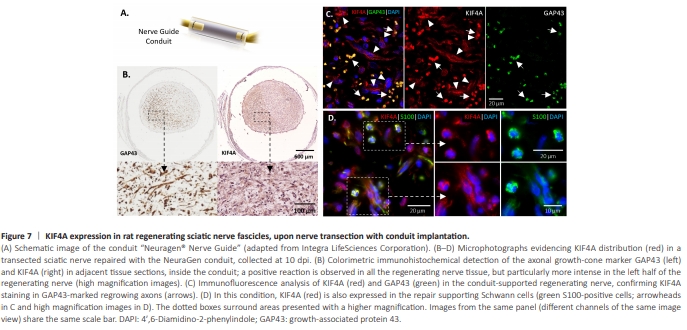
KIF4A has an anterograde transport role in growing axons during development (Peretti et al., 2000; Bisbal et al., 2009), an axonal distribution in the adult uninjured nerve (Figure 3), and Kif4a mRNA levels early increase in regeneration-able injured DRG neurons (Figure 5). Given these, we hypothesized that the newly formed KIF4A protein in the DRG neurons of injured nerves would help to regrow their damaged axons and thus be found in the growth cones of regenerating nerves. To test this, the established nerve implant NeuraGen? Nerve Guide Collagen Type I Conduit (Carriel et al., 2017; Figure 7A) was used to bridge the proximal and distal stumps of a transected rat sciatic nerve. The conduit was further sectioned at 10 dpi, a time when the growth cones of the regrowing proximal axons are within the medial part of the conduit. A distinct fibrous distribution of regenerating axons, with a focus in the left half of the distal cross-section, was observed when these axons were immunostained for the growth cone marker growth associated protein 43 (GAP43) (Figure 7B, left). KIF4A staining at an immediately adjacent section showed a similar distribution (Figure 7B, right). Of note, the observed lateralization of growth cones may be ascribed to distinct phases of nerve regeneration within the conduit due to initial uneven diffusions of proregenerative molecules (Chato-Astrain et al., 2023). An immunofluorescence analysis of KIF4A and GAP43 in an adjacent cross-section revealed a highly abundant KIF4A expression in the GAP43-positive regrowing axons (Figure 7C, arrows). KIF4A signal was observed not only in the ingrown axons, but also in neighboring cells (Figure 7C, arrowheads). To confirm their identity, co-staining of KIF4A and the Schwann cell marker S100 was also performed (Figure 7D). As expected, many cells in the intraluminal regenerating nerve tissue were positive for both S100 and KIF4A, confirming KIF4A abundant expression in repair Schwann cells (Figure 7D, higher magnification images).
Figure 8 | KIF4A role in the proliferation of immature Schwann cells.
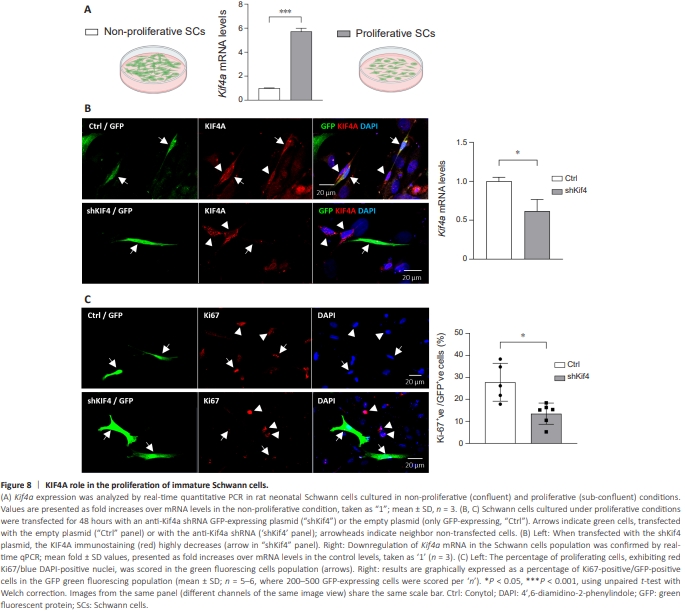
Damage to a peripheral nerve, whether by crush or transection, causes increases in Kif4a expression in both DRG neurons and Schwann cells at 2 dpi. During the first days after injury, myelin and non-myelin (Remak) Schwann cells distal to the nerve injury already undergo a large-scale change into regeneration-support cells (Bosse et al., 2006; Barrette et al., 2010; ArthurFarraj et al., 2012). This switch involves dedifferentiation and reprogramming into a “repair” phenotype, which is accompanied by the gain of a strong cellular proliferation capacity (Arthur-Farraj et al., 2017). Using primary Schwann cell cultures, which are similar to some extent to the immaturelike status of repair Schwann cells (Liu et al., 2015), we further tested if the proliferation activity of cultured Schwann cells correlated with Kif4a expression. To this aim, rat primary Schwann cells were collected when proliferating in sub-confluent cultures (40%–50% density), and when in density-arrested cultures maintained at 100% confluency for 2 days to assure cell contact-inhibited proliferation. Confirming our hypothesis, Kif4a mRNA levels were significantly (~6-fold) higher in proliferating Schwann cells than in non-proliferating ones (Figure 8A). To better characterize these cultures, the levels of two known markers of Schwann cells, Oct6 (SCIP/Tst1) and Krox20 (EGR2) (Liu et al., 2015), were analyzed. These transcription factors that induce pro-myelinating pathways (Svaren and Meijer, 2008; Liu et al., 2015) were highly increased in the confluent non-proliferating Schwann cells (Additional Figure 7A), supporting that these were closer to a differentiated state, and in accordance with the work by Liu et al. (2015). Altogether, these results indicate that Kif4a expression peaks in the more immature proliferating Schwann cells. Given this data and the known KIF4A chromokinesin functions in the mitotic spindle in other cells (Zhu and Jiang, 2005; Samejima et al., 2012), a probable role for Kif4a in Schwann cell proliferation was further tested. Endogenous Kif4a expression was partially suppressed in rat primary Schwann cells grown under proliferative culture conditions and transfected with an anti-KIF4A shRNA GFP-expressing plasmid (Figure 8B and C, plasmid map and Kif4a target sequence in Additional Figure 7B and C). Double KIF4A/ GFP immunocytochemistry labeling (Figure 8B; also Additional Figure 7D) confirmed KIF4A silencing in Schwann cells specifically transfected with the anti-KIF4A shRNA GFP-expressing plasmid (“shKif4a” lower panel, arrow), but not when transfected with the empty GFP vector (“Ctrl” upper panel, arrows) nor in non-transfected cells (arrowheads, GFP-negative cells). About 20% of the Schwann cells were transfected with the shKif4a plasmid, and a real-time qPCR analysis demonstrated that Kif4a expression was silenced by ~45% in the entire population (Figure 8B, graph). Subsequently, the cells were labeled with Ki-67, a nuclear marker of cell proliferation (Figure 8C, microphotograph panels). The percentage of Ki-67/GFP double-positive cells was scored in the GFP-expressing cells population, and the number of proliferating cells was decreased by ~50% in the shKif4a transfected green fluorescing population (Figure 8C, graph). This result confirms our hypothesis of a causal relationship between Kif4a expression and the proliferation of immature-like Schwann cells.