脊髓损伤
-
Figure 1|SRF expression in spinal cord tissue in normal and SCT rats.
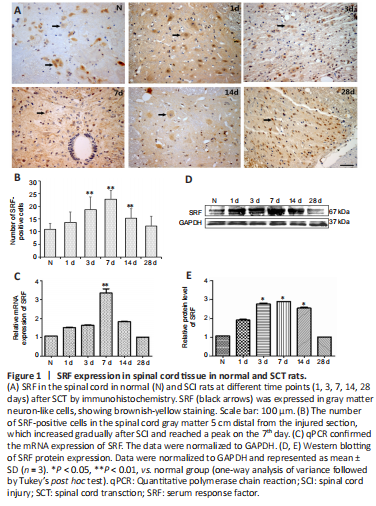
Immunohistochemistry was used to determine the cellular localization and expression of SRF in the spinal cord tissue of rats. SRF was mainly localized at the nucleus and cytoplasm of gray matter neurons in normal and injured spinal cord tissue, and varied at different time points after SCI. Compared with that of the normal group, the number of SRF-positive cells in the SCI group was increased on the 1st day after SCI, reached the maximum on the 7th day, then was decreased on the 14th day, and eventually reached an expression level similar to that of the normal group (Figure 1A and B).
qPCR and western blotting experiments were used to further verify SRF expression in the injured spinal cord, and the results were consistent with those acquired by immunohistochemistry. qPCR, western blotting and immunohistochemistry showed that SRF gradually increased after SCI, and reached the highest level on the 7th day, then gradually decreased, and finally returned to similar to the normal level on the 28th day after SCI (Figure 1C–E).
Figure 2|Identification of lentivirus transfected into PC12 cells and spinal cord tissue.
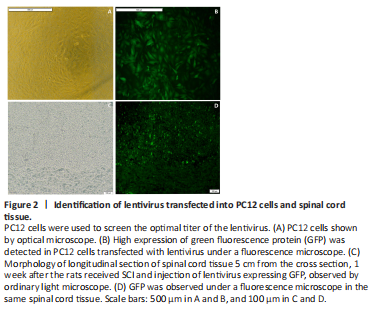
To explore the function of SRF in SCI rats, human immunodeficiency virus-based vectors, the most popular and effective lentivirus-based expression system, were used. First, transfected PC12 cells were applied to screen the optimal titer of the lentivirus by considering the cell status and fluorescence intensity (Figure 2A and B). Then, verification for viral transfection in vivo was performed. One week after injection of GFP-labeled LV-srf into the spinal cord tissue, a high expression of green fluorescence was detected under a fluorescence microscope (Figure 2C and D). qPCR and western blotting were used to determine the difference of SRF expression between the NC and normal groups, and showed that the empty vector had no significant effect on SRF expression level. Compared with the NC and normal groups, the SRF overexpressed and silenced groups had significant differences in SRF expression level (Figure 3).
Figure 5|SRF regulates axonal regeneration after SCI.
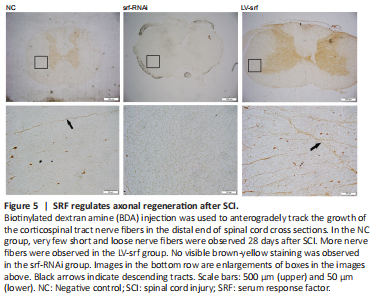
To investigate the growth of nerve fibers after SCT, BDA injection was used to anterogradely track the growth of corticospinal tract nerve fibers. Very few short and loose nerve fibers were observed in the spinal cord 14 days after BDA injection in the NC group. More nerve fibers with a certain length and density were observed in the LV-srf group, whereas no visible brown-yellow staining was observed in the srf-RNAi group, suggesting that an increase of regenerated nerve fibers was associated with increased SRF expression (Figure 5).
Figure 6|SRF promotes axon regeneration after SCI, shown by electron microscope.
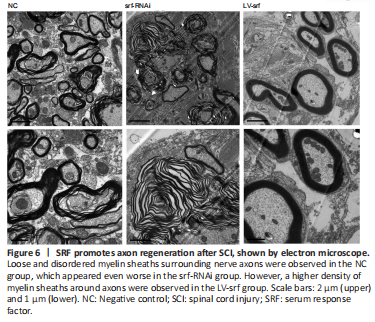
We also observed the myelin sheaths surrounding axons by electron microscope. Loose and disordered myelin sheaths surrounding nerve axons were observed in the NC group, and abundant extracellular matrix was observed in the white matter. Severe demyelination was found in the srf-RNAi group, whereas, in the LV-srf group, a higher density of myelin sheath was observed around axons, mitochondrial structure in neurons was relatively complete, and the cross sections of the myelin sheath were roughly consistent with the neuron axon (Figure 6). These results suggest that SRF may promote axon regeneration.
Figure 7|Overexpressed SRF accompanied by upregulated GAP43 after SCI.
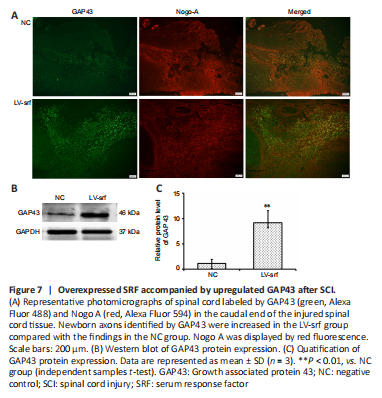
To further investigate the relationship between SRF overexpression and axon regeneration, we performed immunofluorescence to detect GAP43 expression after SCI. Compared with those in the NC group, axons in the LV-srf group had noticeably increased GAP43 expression (Figure 7A). This increase in GAP43 protein expression was quantified by western blot assay (Figure 7B and C). Additionally, axon growth inhibitor, Nogo A expression was displayed by double immunostainingwith GAP43 expression (Figure 7A). Taken together, the results suggest that newborn axons were present at the lower end of the injured spinal cord in SCI rats with SRF overexpression.