脊髓损伤
-
Figure 1|Depletion of Fign improves functional recovery by increasing Tyr-tubulin.

Axonal regeneration is limited in the central nervous system but is available for regeneration in the peripheral nervous system. A previous study reported that after injury of the peripheral nervous system, axonal regeneration was promoted by increasing tubulin dynamics (Cho and Cavalli, 2012). We previously found that Fign knockdown notably increased Tyr-tubulin mass (Hu et al., 2017); therefore, we hypothesized that differences in labile Tyr-MTs (or Tyr-tubulin) may be an important contributing factor. Thus, we established rat models of SCI and sciatic nerve injury, and compared protein alterations in Tyr-tubulin and Fign protein levels by western blotting at 3, 7, 14, 21, and 28 days after injury. Our results show that after SCI (Figure 1A) or sciatic nerve injury (Figure 1B), Tyr-tubulin increased continuously. The level of Fign decreased slightly after SCI, whereas it was notably decreased after sciatic nerve injury.
These results show that compared with the spinal cord, Tyr-tubulin protein is considerably more abundant in the sciatic nerve. Even after injury, Tyr-tubulin protein showed a rapid increase, which may contribute to peripheral nerve regeneration. In addition, a previous report found that Fign depletion enhanced DRG neuronal regeneration (Matamoros et al., 2019). Thus, we sought to determine whether Tyr-tubulin augmentation due to Fign knockdown contributes to improvement after SCI.
We established the spinal cord contusion model in adult rats as described in our previous study (Zhao et al., 2017). AAV9-U6-shFign was injected to allow Fign depletion (Figure 1C and D). After injury, we used the BBB score to evaluate behavioral recovery of the rats. Our results show that Fign knockdown improved BBB score, which reached significance on the 14th day post-injury (Figure 1E). We also confirmed Fign knockdown efficiency at 14 days post-injury by western blotting (Figure 1F). Next, we observed the effects of Fign knockdown by immunohistochemistry at 14 days post-injury. In the Fign-shRNA treated group (Figure 1G and H), immunopositivity of NF200 was markedly increased in the injured area. These results support our assumption that Fign knockdown improves functional recovery and enhances axonal regeneration after SCI. Interestingly, we found that knockdown of Fign resulted in a significant increase in the MT plus-end binding protein, EB3, which was mostly concentrated in Tyr-tubulin enriched areas (Figure 1I and J). Our previous study reported that Fign preferentially works on Tyr-tubulin (Hu et al., 2017) and that EB3 usually binds at the growing plus-end of MTs (Qu et al., 2019). These newly added tubulins are tyrosinated. This raises the question of whether Fign is transported to Tyr-MTs via an interaction with EB3.Figure 2|Fign knockdown increases EB3 entry into growing neurites.
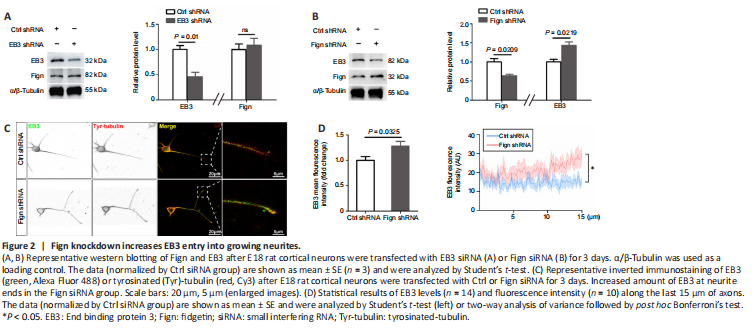
Next, we investigated whether Fign depletion affects EB3 expression or localization. First, we confirmed the knockdown efficiency of Fign-siRNA (Additional Figure 1A and B). We then confirmed that Fign siRNA-treated neurons displayed notably longer axons and more branches than neurons under the control (Ctrl) siRNA treatment (Additional Figure 1C and D). This phenomenon is consistent with our observation of an increase in NF200 following Fign depletion after SCI. To further examine the relationship between Fign and EB3 expression, we depleted EB3 or Fign using RNA interference and examined changes in their expression. Our results showed that depletion of EB3 did not change expression of Fign (Figure 2A). Conversely, depletion of Fign significantly increased expression of EB3 (Figure 2B). Depletion of Fign increased the amount of EB3 at the end of neurites (Figure 2C). We further analyzed the fluorescence intensity and distribution of EB3 at the distal (15 μm) neurite ends. These results showed that EB3 increased significantly at the distal end of growing neurites after Fign depletion (Figure 2D). This suggests that depletion of Fign results in augmentation of the Tyr-tubulin mass by promoting EB3 entry into growing neurites.
Figure 3|Fign interacts with EB3 leading to more Tyr-tubulin moving through the T-zone into the P-domain.
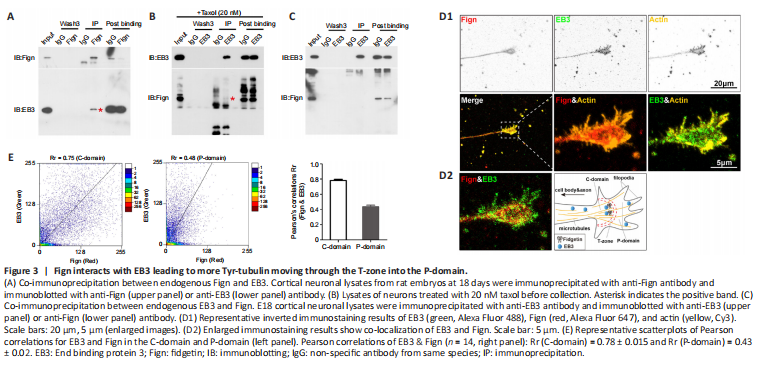
Based on the above findings, we next performed endogenous co-immunoprecipitation assays to confirm an interaction between Fign and EB3. Fign antibody pulled-down EB3 protein (Figure 3A), whereas EB3 antibody pulled-down Fign when taxol (20 nM) was pre-added to the cell culture medium (Figure 3B). Without taxol, the interaction between Fign and EB3 was not detected (Figure 3C). This indicates that most Fign is bound to EB3, with only a tiny amount of EB3 bound to Fign. Tyr-MTs are enriched at axonal ends and low doses of taxol leads to EB3 accumulation at the tips of neurites (Witte et al., 2008). Thus, we propose that treatment with a low dose of taxol must increase the chances of EB3 interacting with Fign. Next, we analyzed localization of Fign, EB3, and actin by immunofluorescence staining (Figure 3D1). We found co-localization of EB3 and Fign at the axonal shaft and C (central)-domain of growth cones (Figure 3D2). Pearson correlation showed distribution of these two proteins at the C-domain and P (peripheral)-domain (Figure 3E). Together, these results suggest that the Fign/EB3 complex is distributed along the axonal shaft and restricted to the T (transition)-zone. Thus, depletion of Fign enables greater amounts of Tyr-tubulin to break through the T-zone into the P-domain.
Figure 4|Fign regulates Tyr-tubulin by binding to EB3 and parking at microtubules plus-ends.
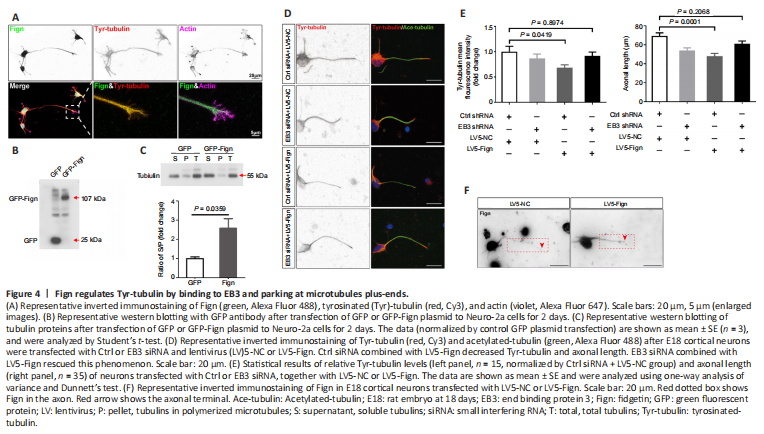
Next, we wanted to determine whether Fign regulates Tyr-MTs through its interaction with EB3. First, colocalization of Fign with Tyr-tubulin was verified by immunostaining (Figure 4A), revealing that Fign prefers to dock at Tyr-tubulin enriched MTs. Usually, tubulin newly added to the plus-end of MTs are tyrosinated. Therefore, we proposed that Fign overexpression may cause more Tyr-tubulin to move from MTs into the cytosol, resulting in more Tyr-tubulin (or free tubulin) in the supernatant. We next performed the polymerized tubulin assay, as previously described (Xu et al., 2018). Our results showed that Fign-GFP overexpression increased soluble tubulin (in the supernatant) and decreased polymerized tubulin in the pellet (Figure 4B and C). The ratio of supernatant to pellet tubulin increased to 257% after Fign overexpression. These findings suggest that Fign overexpression retained more unassembled Tyr-tubulin and free tubulin in the supernatant. In contrast, depletion of Fign notably increased Tyr-tubulin, especially at the distal end of the growing axon (Additional Figure 2A).
We further tested whether removal of Tyr-tubulin abolishes the function of Fign. Neurons and astrocytes were cultured and treated with Fign siRNA using the same procedure as in our previous studies (Hu et al., 2017; Ma et al., 2023). With increasing taxol (Additional Figure 2B), MTs became concentrated, whereas Tyr-tubulin was significantly reduced and acetylated (Ace)-tubulin increased (Additional Figure 2C). As expected, Fign siRNA increased Tyr-tubulin mass (Additional Figure 2D), whereas taxol eliminated this effect. However, when taxol was removed, Fign depletion restored the increase of Tyr-tubulin. Similarly, taxol application eliminated the increase in Tyr-tubulin in Fign siRNA-treated neurons (Additional Figure 2E).
To confirm the function of the interaction between Fign and EB3, we deleted EB3 by RNA interference in combination with LV5-Fign viral expression to examine the effects on Tyr-tubulin and axonal length. Fign overexpression decreased Tyr-tubulin and axonal length (Figure 4D and E), with the effects eliminated after EB3 depletion. Figure 4F showed increased LV5-Fign resulted in more Fign in axons. These results show that Fign trimmed Tyr-tubulin via interaction with EB3.