NRR:南通大学刘梅团队提出Fidgetin是促进损伤轴突再生的新靶点
成年哺乳动物脊髓损伤后轴突的再生仍然是一个极具挑战的临床问题。提高轴突再生能力的策略在神经科学中具有重要的意义。通过不同的方法操纵细胞骨架动力学被认为是促进神经修复的有效策略[1]。神经元是具有特殊极性形态的有丝分裂后细胞,可利用微管阵列构建其轴突和树突。而微管主要参与了维持神经元极性、轴突运输、轴突生长、分支形成、生长锥导航和突触形成[2]。脊髓损伤后,轴突神经网络受损,且轴突微管逆行解聚在轴突远端形成缩回球,这阻碍了轴突的再生[3, 4]。临床研究发现,大多数脊髓损伤患者仍保留残余的轴突[5, 6],但由于微管重构能力受限导致其损伤轴突无法再生[7]。因此,增强微管动态重构能力以促进轴突再生的策略,对于开发治疗神经损伤新方法具有重大意义[8]。
来自中国南通大学刘梅团队最近在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Fidgetin interacting with microtubule end binding protein EB3 affects axonal regrowth in spinal cord injury”的研究,比较了脊髓损伤和周围神经损伤后不稳定微管的表达和微管切割蛋白Fidgetin的表达模式,并测试了改变微管修饰水平促进脊髓损伤后轴突再生的可行性。这一研究不仅表明Fidgetin是一个促进损伤后轴突再生的新治疗靶点,而且揭示Fidgetin与微管末端结合蛋白3(end binding protein 3,EB3)相互作用是其靶向不稳定微管的机制。
轴突再生在中枢神经系统中极为罕见,但其却能出现在周围神经系统中。此前有研究报道称,周围神经系统损伤后,可通过增强微管动力来促进轴突再生[9]。因此刘梅等认为不稳定的酪氨酸化微管的差异可能是影响轴突再生的重要因素。既往研究发现,敲除Fidgetin可显著增加酪氨酸化微管蛋白的质量[10]。因此该实验建立了脊髓损伤和周围神经(坐骨神经)损伤大鼠模型,发现与脊髓损伤相比,周围神经损伤后不稳定微管中酪氨酸化微管蛋白持续升高,而Fidgetin水平则明显下降(图1)。因此,刘梅等通过脊髓注射AAV9-U6-shFign病毒敲低Fidgetin,发现脊髓损伤大鼠的BBB评分有显著改善(图1)。有趣的是,敲低Fidgetin会导致微管正末端结合蛋白EB3的增加,且主要集中在酪氨酸化微管蛋白富集区域(图1)。由于刘梅等以往曾发现Fidgetin优势作用于酪氨酸化微管蛋白,而EB3是一种结合在生长中微管正末端的蛋白。因此,新聚合的微管是酪氨酸化修饰的。据此引出一个问题,即Fidgetin是否可通过与EB3的相互作用从而靶向酪氨酸化的微管?
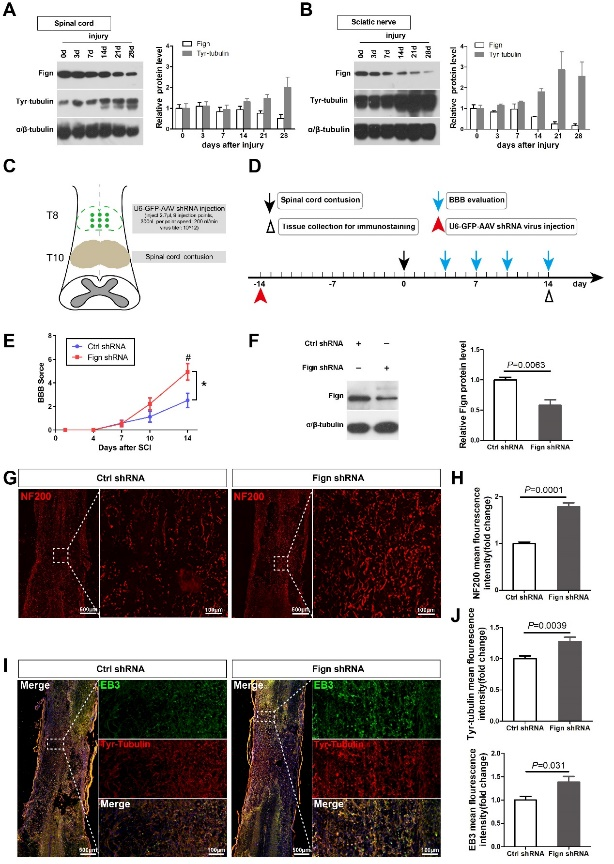
图1与脊髓损伤相比,周围神经损伤后不稳定微管酪氨酸化微管蛋白持续升高,而Fidgetin水平则明显下降。干预Fidgetin表达可改善脊髓损伤大鼠的BBB评分。有趣的是,敲低Fidgetin不仅增加了酪氨酸化微管蛋白,而且导致微管正末端结合蛋白EB3显著增加,且主要集中在酪氨酸化微管蛋白富集区域(图源:Ma et al., Neural Regen Res, 2023)
接下来,刘梅等为进一步揭示Fidgetin与EB3的表达关系,采用RNA干扰方法敲低EB3或Fidgetin。结果可见,EB3缺失没有改变Fidgetin表达,但Fidgetin缺失却可显著增加EB3的表达和分布(图2)。研究进一步分析了EB3在轴突远端15 µm处的荧光强度和分布情况,发现Fidgetin敲低后,EB3在生长的轴突远端明显增加(图2)。这一结果提示,Fidgetin缺失可释放更多的EB3进入生长中的神经突起,从而增加酪氨酸化微管蛋白水平。
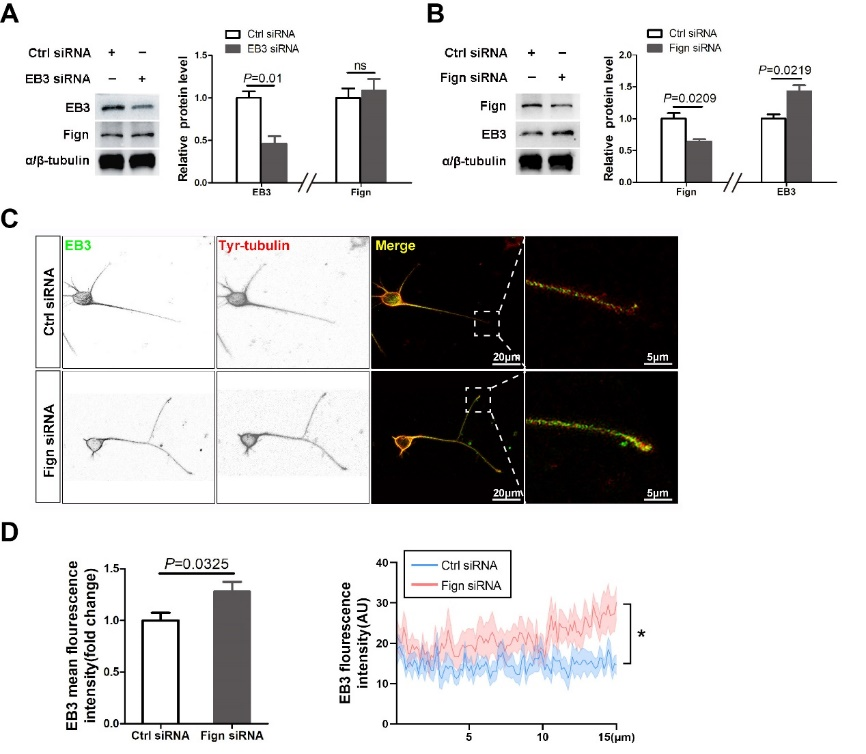
图2 敲低Fidgetin可显著增加EB3蛋白表达以及在神经突起末端分布(图源:Ma et al., Neural Regen Res, 2023)
基于上述结果,采用内源性免疫共沉淀方法证实了Fidgetin与EB3的相互作用(图3)。且免疫荧光染色结果也验证了Fidgetin、EB3和actin的定位关系,发现EB3与Fidgetin共定位于生长锥的C区(图3)。随后阐明了Fidgetin是否可通过与EB3相互作用来调控酪氨酸化微管蛋白。结果显示Fidgetin更倾向于停靠在酪氨酸化微管蛋白富集的微管上。Fidgetin 过表达可导致细胞上清液中保留了更多未组装的游离微管蛋白。为证实Fidgetin与EB3相互作用关系,实验敲除EB3的同时过表达Fidgetin以观察对酪氨酸化微管蛋白和轴突长度的影响,结果显示, Fidgetin在轴突中显著增加,同时酪氨酸化微管蛋白和轴突长度则明显减少,但该现象在敲除EB3后消失(图4)。据此认为,Fidgetin可通过与EB3结合靶向正在生长中的微管末端,修剪酪氨酸修饰的微管。而当缺少Fidgetin时,微管不稳定区延长从而增加轴突的长度和分支数量,因此Fidgetin可作为调节微管骨架重构促进轴突再生的靶标分子。

图3 Fidgetin缺失可导致更多的酪氨酸化微管蛋白突破T区进入P区,促进轴突的延伸(图源:Ma et al., Neural Regen Res, 2023)
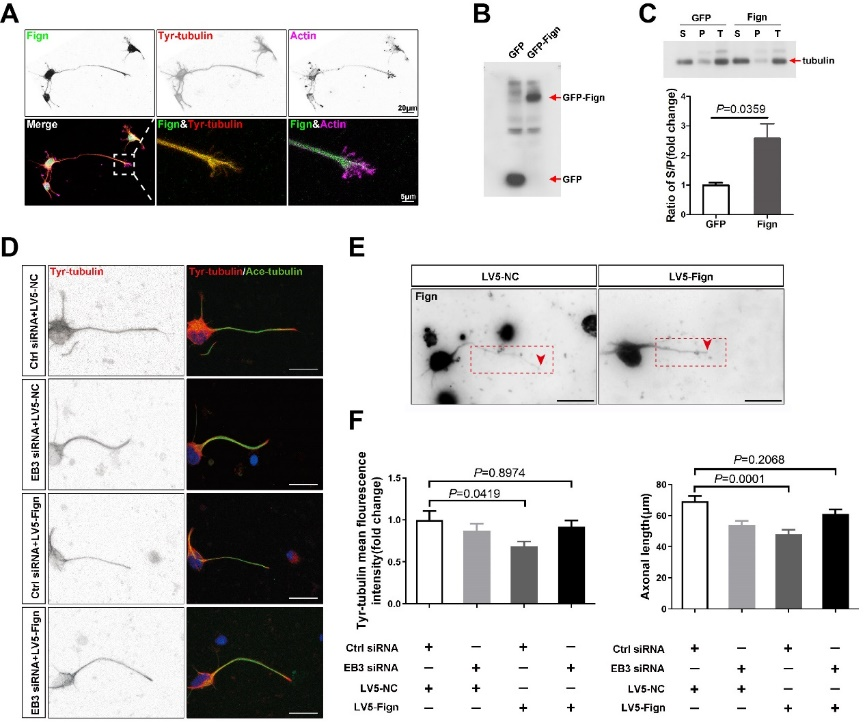
图4Fidgetin修剪酪氨酸化修饰微管的作用是通过EB3完成的(图源:Ma et al., Neural Regen Res, 2023)
综上所述,刘梅等首次报道了Fidgetin与EB3存在相互作用,解释了Fidgetin作用于不稳定修饰的酪氨酸化微管的机制:即微管正端结合蛋白EB3可与Fidgetin相互作用,从而靶向轴突生长的微管正末端,从而修剪新聚合的酪氨酸修饰的微管。Fidgetin的作用是修剪微管正末端,在轴突生长中助于正确的寻路和适当的分支。因此,敲低Fidgetin后,新聚合的微管蛋白继续加入延长了微管的不稳定端,从而延长轴突,增加分支。
但是尚不清楚Fidgetin与EB3的结合是直接的还是有其他分子参与的,因而未来可考虑蛋白结构域截短体构建和GST-pull down等方法进一步分析来解析Fidgetin与EB3的互作结构域,或者找寻其他可能在Fidgetin与EB3之间发挥作用的分子。
原文链接:https://doi.org/10.4103/1673-5374.373716
参考文献
[1] Blanquie O, Bradke F. Cytoskeleton dynamics in axon regeneration. Curr Opin Neurobiol. 2018;51:60-69.
[2] Matamoros AJ, Baas PW. Microtubules in health and degenerative disease of the nervous system. Brain Res Bull. 2016;126(Pt 3):217-225.
[3] Hur EM, Saijilafu, Zhou FQ. Growing the growth cone: remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci. 2012;35(3):164-174.
[4] Sofroniew MV. Dissecting spinal cord regeneration. Nature. 2018;557(7705):343-350.
[5] Fawcett JW, Curt A, Steeves JD, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45(3):190-205.
[6] Lasiene J, Shupe L, Perlmutter S, et al. No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci. 2008;28(15):3887-3896.
[7] Kulkarni R, Thakur A, Kumar H. Microtubule dynamics following central and peripheral nervous system axotomy. ACS Chem Neurosci. 2022;13(9):1358-1369.
[8] Anderson MA, O'shea TM, Burda JE, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561(7723):396-400.
[9] Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31(14):3063-3078.
[10] Hu Z, Feng J, Bo W, et al. Fidgetin regulates cultured astrocyte migration by severing tyrosinated microtubules at the leading edge. Mol Biol Cell. 2017;28(4):545-553.



