视神经损伤
-
Figure 3|Conditional knockout of ZnT3 in ACs inhibits Zn2+ increase in the retina after injury.
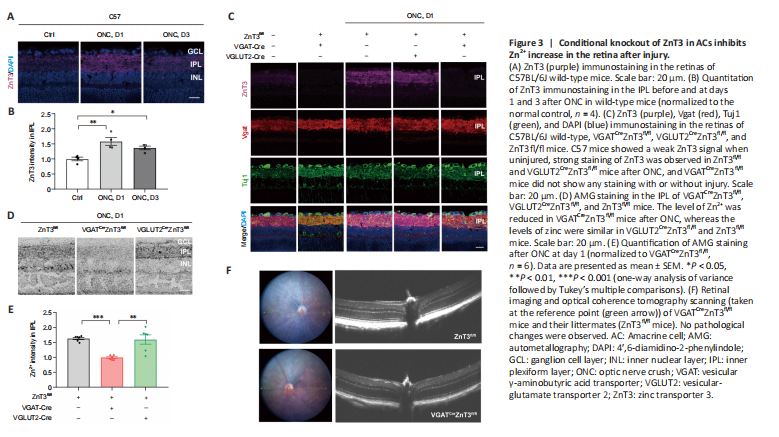
We first assessed the expression levels of ZnT3 and Zn2+ in mouse strains after ONC. In intact mice, a faint ZnT3 signal in the IPL was observed, and it significantly increased (P = 0.003) by the first day after ONC; on the third day, ZnT3 level decreased but was still significantly higher (P = 0.0402) than that in controls (Figure 3A and B). This is consistent with our previous study (Li et al., 2017), which showed that Zn2+ levels were highest in the IPL on the first day after injury. We therefore next examined the expression of ZnT3 at 1 day post-ONC. On the first day after ONC, strong staining of ZnT3 was observed in littermate control (ZnT3fl/fl) and VGLUT2CreZnT3fl/fl mice; however,
VGATCreZnT3fl/fl mice did not show ZnT3 expression with or without injury (Figure 3C). These results suggest that ZnT3 accumulates primarily in vesicles in ACs. This was supported by the strong overlap between ZnT3 and vesicular γ-aminobutyric acid transporter VGAT, an AC synapse marker, but not Tuj1, an RGC marker (Figure 3C).
Autometallography was performed to visualize mobile zinc in the IPL because of its selectivity for Zn2+ (Danscher and Stoltenberg, 2005). While the control group (ZnT3fl/fl) and VGLUT2CreZnT3fl/fl mice (conditional knockout of ZnT3 in RGCs) showed similar levels of zinc after ONC, VGATCreZnT3fl/fl mice, with ZnT3 deletion in ACs, showed lower levels of Zn2+ (Figure 3D and E). Thus, these results reveal that selective deletion of ZnT3 in ACs resulted in reduced Zn2+ after injury. No architectural pathological changes were observed in the transgenic mice via fundus color photography and optical coherence tomography (Figure 3F), demonstrating that the gene knockout did not affect the normal structural features of the retina.
Figure 4|Knockout of ZnT3 in ACs promotes RGC survival.

The release of several cytokines contributes uniquely to the process of neuroinflammation. Because of this, we investigated the production of a variety of cytokines related to Aβ deposits, including IL-1β, IL-6, and TNF-α, as well as the anti-inflammatory cytokine, IL-10. Compared with the WT+CD group, production of IL-1β (P < 0.001), IL-6 (P < 0.01), and TNF-α (P < 0.05) were considerably increased in both the cortex and hippocampus of APP/PS1 mice treated with CD. However, the KD therapy group showed a significant reduction in IL-1β, IL-6, and TNF-α levels in both the cortex and hippocampus (P < 0.05 for each variable). The Luminex test showed a significant increase in IL-10 production in the cortex (P < 0.05). MCP-1/CCL2 is one of the primary chemokines that controls the recruitment and activation of monocytes and microglia (Zhang and Luo, 2019). KD therapy resulted in a substantial decrease in the levels of cortical MCP-1 (P < 0.05). This suggests that KD may be able to reduce neuroinflammation in the cerebral cortex and hippocampus of APP/PS1 animals (Figure 4A and B).