NRR:中山大学眼科中心卓业鸿和李轶擎团队发现选择性敲除无长突细胞中锌转运蛋白3可促进视网膜神经节细胞存活和视神经再生
撰文:刘哲
视神经由视网膜神经节细胞的轴突组成,是中枢神经系统中最重要的结构之一。然而视网膜神经节细胞一旦受伤就会失去再生轴突的能力,进而导致不可逆的视野缺失[1]。除细胞内在机制以及由胶质细胞、炎症细胞构成的细胞外环境外,视网膜神经节细胞命运也受到视网膜的复杂环路和多细胞级联信号激活的影响,包括抑制性中间神经元的变化,如无长突细胞[2, 3]。来自中国中山大学中山眼科中心卓业鸿和李轶擎团队既往研究已证明视神经损伤后视网膜内丛状层中游离锌(Zn2+)迅速增加,并可抑制视网膜神经节细胞的存活和再生[4]。锌转运蛋白3可将Zn2+装载到突触囊泡中,因而可间接显示细胞间锌水平[5]。免疫荧光染色可见,锌转运蛋白3与无长突细胞的强烈共定位,提示锌转运蛋白3包裹Zn2+进入了突触囊泡,并从无长突细胞末端释放,引起视网膜神经节细胞的死亡[4]。然而,突触锌的细胞定位及其在无长突细胞和视网膜神经节细胞之间的神经递质作用的直接证据并未得到证明。
近期,卓业鸿等在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Selective deletion of zinc transporter 3 in amacrine cells promotes retinal ganglion cell survival and optic nerve regeneration after injury”的研究,其分别通过在无长突细胞和视网膜神经节细胞中条件性敲除锌转运蛋白3,构建了2种转基因小鼠。结果发现,视神经损伤后,视网膜中急剧升高的游离Zn2+来源于无长突细胞,而在无长突细胞中选择性敲除锌转运蛋白3能够有效促进视神经钳夹伤后视网膜神经节细胞的存活以及视神经的再生,并能改善视网膜神经节细胞功能,促进视力恢复。对视网膜神经节细胞进行测序分析结果提示,抑制突触前Zn2+的释放能够影响突触后神经元视网膜神经节细胞内存活相关关键基因的转录,调节无长突细胞-视网膜神经节细胞突触间联系,从而影响视网膜神经节细胞的命运。这项研究为视神经,甚至是神经元损伤的修复提供了新的见解和思路。
视神经损伤后,视网膜内丛状层中Zn2+水平迅速升高,但Zn2+细胞来源定位尚不明确[4]。在此研究中,卓业鸿等分别在无长突细胞和视网膜神经节细胞中条件性敲除锌转运蛋白3构建了2种转基因小鼠(VGATCreZnT3fl/fl和VGLUT2CreZnT3fl/fl),以明确Zn2+的细胞定位。可见无长突细胞中特异性敲除锌转运蛋白3能够抑制损伤后Zn2+水平上升,这提示锌来源于中间神经元中无长突细胞(图1),且通过在无长突细胞中条件性敲除锌转运蛋白3抑制突触前Zn2+释放能够促进视网膜神经节细胞存活和视神经再生(图2),这与卓业鸿等等之前得出的锌离子螯合剂对视网膜神经节细胞的保护作用这一结果相符[4]。在此项研究中,他们还验证了在无长突细胞中特异性敲除锌转运蛋白3改善视网膜神经节细胞功能以及保护视力的效果。流式分选视网膜神经节细胞测序分析提示,此现象的可能分子机制为调控无长突细胞内突触前Zn2+的释放能够影响突触后视网膜神经节细胞内存活相关基因的转录,并调节无长突细胞-视网膜神经节细胞突触可塑性和突触间交流,从而影响视网膜神经节细胞的存活和视神经的再生(图3)。
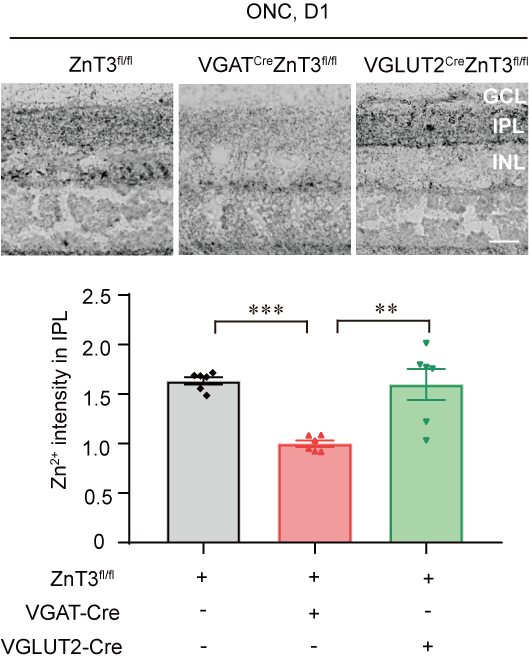
图1 视神经损伤小鼠模型中选择性敲除无长突细胞中锌转运蛋白3(VGATCreZnT3fl/fl)能够抑制内丛状层Zn2+的升高(图源:卓业鸿、李轶擎实验室)

图2在无长突细胞中特异性敲除锌转运蛋白3(VGATCreZnT3fl/fl)可促进视神经损伤后视网膜神经节细胞的存活和视神经的再生(图源:卓业鸿、李轶擎实验室)
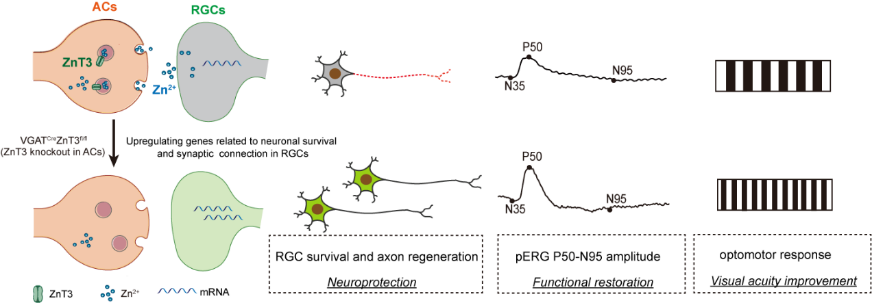
图3在无长突细胞中特异性敲除锌转运蛋白3可通过影响视网膜节细胞内关键基因的转录促进损伤后节细胞存活和视神经再生,并改善视网膜神经节细胞功能以及视力水平(图源:卓业鸿、李轶擎实验室)
综上所述,来自无长突细胞突触前Zn2+对视网膜神经节细胞的存活和视神经的再生有着负性调节作用。卓业鸿等的研究揭示了中间神经元可通过释放适当的神经化学物质(如锌)来影响其靶神经元的活动,且正如Paul等[6]的研究,锌是神经元信号的有效调节剂。此次研究中2种不同类型的条件敲除小鼠为锌来源的细胞定位提供了更直接且强有力的证据,这使锌依赖性视网膜神经节细胞死亡的理论更加准确和完整,也为复杂的视网膜细胞网络间作用提供新的见解。但是这一发现还需青光眼慢性模型等多种模型的验证,且对分选视网膜神经节细胞的测序分析并未明确到某一具体的分子机制通路,因而还需进一步探索。
原文链接:https://doi.org/10.4103/1673-5374.373660
参考文献
[1] Williams PR, Benowitz LI, Goldberg JL, et al. Axon regeneration in the mammalian optic nerve. Annu Rev Vis Sci. 2020;6:195-213.
[2] Sergeeva EG, Rosenberg PA, Benowitz LI. Non-cell-autonomous regulation of optic nerve regeneration by amacrine cells. Front Cell Neurosci. 2021;15:666798.
[3] Kim T, Soto F, Kerschensteiner D. An excitatory amacrine cell detects object motion and provides feature-selective input to ganglion cells in the mouse retina. Elife. 2015;4:e08025.
[4] Li Y, Andereggen L, Yuki K, et al. Mobile zinc increases rapidly in the retina after optic nerve injury and regulates ganglion cell survival and optic nerve regeneration. Proc Natl Acad Sci U S A. 2017;114(2):E209-E218.
[5] Palmiter RD, Cole TB, Quaife CJ, et al. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci U S A. 1996;93(25):14934-14939.
[6] Paul A, Crow M, Raudales R, et al. Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell. 2017;171:522-539.e20(3).

第一作者:刘哲。
通讯作者:卓业鸿,“珠江学者”特聘教授,广东省特支计划百千万领军人才。从事眼科学临床、科研、教学近三十年,在青光眼早期诊断、药物及手术治疗方面积累了丰富的临床经验。在国内率先开展青光眼家系遗传学研究、成功构建灵长类动物青光眼模型及干细胞移植替代治疗、搭建青光眼临床转化研究平台。
通讯作者:李轶擎,曾在哈佛医学院、波士顿儿童医院从事博士后研究,并受聘哈佛医学院讲师(眼科学与神经外科学)。获“广东省杰出青年医学人才”,致力于青光眼视神经损伤、修复与再生机制及靶向治疗;眼病动物模型构建、视觉功能检测与行为学评估;视觉障碍人群视功能康复与新型辅具产品研发应用方向。
基金支持:国家重点研发计划项目(2020YFA0112701,卓业鸿),国家自然科学基金项目(82171057,卓业鸿;81870657,李轶擎),广东省自然科学基金项目(2022A1515012168,李轶擎) ,广州市科技重点研发计划项目(202206080005,卓业鸿)



