脊髓损伤
-
Figure 1|Human fetal spinal cord BMPRII immunostaining at gestational ages of 15–16 weeks.

Positive immunoreactivity for BMPRII was observed in second-trimester spinal cord age-typed cryosections at approximately gestational ages of 15–16 weeks (Figure 1). BMPRII expression was primarily observed in dorsal (Figure 1A) and ventral horn (Figure 1B) interneurons. The dorsal columns and central grey were negative. Immunoreactivity was also observed along the posterior edge of the ventral funiculus (Figure 1C). In addition, where BMPRII localized to the developing vascular network, present throughout the parenchyma, expression was relatively high (Figure 1D–F), including within the meningeal vasculature (Figure 1G). Supplemental staining of the second-trimester human fetal brain exhibited a similar staining pattern of vascular tubes and scattered single cells (Figure 2A–C).
Figure 2|BMPRII+ hNPCs (red) can also be identified in the human fetal brain cortex (A–C) and are identified by a lack of vascular marker expression (D–H).

In addition to morphology, co-staining with CD34 (Figure 2D) and the ecto-ADPase CD39 (Figure 2E) supported the endothelial origin of the vascular network. In sections of the second-trimester fetal spinal cord, punctate staining of BMPRII+ cells (Figure 2G) colocalized in vessels co-positive for these markers (Figure 2H), whereas the single scattered BMPRII+/CD34–/CD39– cells resided in the tissue parenchyma (Figure 2H, white arrow) which sometimes localized in the vicinity of blood vessels (Figure 2H, yellow arrow). The extent and density of BMPRII+ vasculature within the spinal cord parenchyma are similar to the primordial vessels formed via vasculogenesis previously reported between gestational ages of 14–18 weeks in human fetal retinal development (Hughes et al., 2000). The vessels’ caliber is uniform, has minimal branching, and lacks adjacent capillary plexus formed by angiogenesis and driven by metabolically active HIF1α-VEGF165 expression we observed in later embryonic development.
Figure 3|Human fetal spinal cord LIFR and LIF immunostaining at gestational ages of 14–15 weeks.

Positive immunoreactivity for LIF was observed in cryosections of the second-trimester spinal cord of estimated gestational ages of 14–15 weeks (Figure 3 upper). LIF expression was primarily observed in dorsal sensory and ventral horn motor interneurons (identified by labeling, Figure 3, inset A). The dorsal columns, lateral and ventral funiculus did not show immunoreactivity. LIFR staining showed a similar staining pattern to LIF (Figure 3 lower and inset B). BMPRII ligand BMP4 showed the greatest immunopositivity in the meninges (Additional Figure 1C), and vascular tubes scattered throughout the parenchyma (Additional Figure 1B). In non-vascular cells, low immunopositivity was observed in the dorsal horn region (Additional Figure 1A) but only a few single scattered cells in the ventral horns.
Figure 4|LIF positively regulates neurosphere growth as a function of passage number.
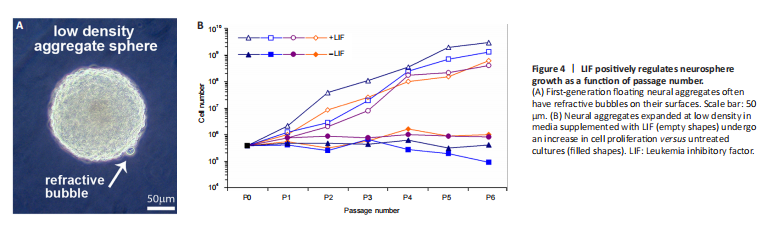
Fetal spinal cords were dissociated and cultured at low density (2.5 × 104 cells/mL in serum-free media) where they proliferated, forming aggregates that floated in suspension with small refractive bubbles along their surface; an observation we have previously noted during the successful expansion of primary neural stem cell cultures (Figure 4A). Because human neural aggregates passaged and seeded at low-density routinely undergo replicative senescence (Svendsen et al., 1998), we set out to examine and quantify factors influencing sphere size and composition and population longevity in culture. LIF is a known mitogen for neural precursors and can prolong the expansion of multipotent neural progenitor cells in culture (Carpenter et al., 1999). Expanded aggregates in LIF-containing media increased overall cell proliferation of second-trimester spinal cord-derived neural precursor cells versus control cultures (Figure 4B, n = 4 donor lines). Consequently, we utilized LIF throughout further experiments.
Figure 5|Effect of culture conditions and LIF prolong culture expansion over repeated passages, while maintaining tripotentiality.
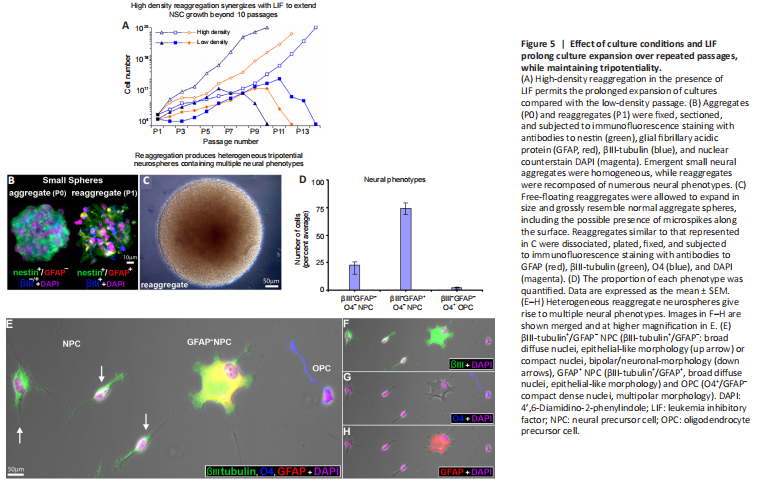
Figure 6|Immunocharacterization of organotypic reaggregate neurospheres.

The process of low-density (2.5 × 104 cells/mL) passage (trypsinization followed by mechanical dissociation) often leads to senescence beyond 8 passages (Figure 5A, n = 3 donor lines/condition). Physically cutting neurospheres into more miniature “pies” has been shown to prolong proliferation by maintaining cell-cell contacts. While this alleviated the issue of senescence, we found that the composition of the daughter spheres generated from this process varied such that both the cellular constituents as well as the neurospheres were heterogeneous. To overcome these obstacles, we employed the reaggregation tissue culture technique, which simply involves resuspending cells at a high density (here 2.5 × 106 cells/mL) (Layer et al., 2002). This provides both the benefits of cell-cell contact-signaling as well as facilitating a homogenous redistribution of the cells throughout the media, thereby increasing the likelihood of producing uniform populations from heterogeneous neurospheres. High-density reaggregation in the presence of LIF clearly promoted sustained growth of the neurospheres beyond 10 passages, whereas long passage of low density aggregates showed terminated growth (Figure 5A).
We utilized multiple-marker immunocytochemical staining of cryosectioned neurospheres using cell-specific markers to characterize the cell types found in first-generation aggregates. The majority (> 92 ± 3%) of cryosectioned emergent spheres (≤ 200 μm in diameter) homogeneously expressed nestin and βIII-tubulin, and were not observed to express GFAP at that size (Figure 5B, aggregate). In larger neurospheres (approximately 400 μm diameter), a similar pattern of homogenous immunopositivity was seen with GFAP, suggesting the possibility of lineage elaboration and/or that human neural precursors begin to express GFAP when physically constrained within large neurospheres (Figure 6A and B, merged in 6C).
High-density reaggregate cultures generated symmetrical spherical cell clusters that grossly resembled neurospheres formed under low-density culture conditions. Our findings also showed that reaggregate spheres form relatively quickly and are already heterogeneous (Figure 5B). Reaggregates quickly develop into large spheres which grossly resemble those in low-density aggregate culture and formed microspikes on the outermost strata of cells (Figure 5C), a feature we commonly observe in low-density cultures. Once plated, reaggregate spheres similarly generated the multiple neural phenotypes we had previously characterized emerging from human spinal cord-derived (low-density) neurospheres (Weible and Chan-Ling, 2007). When plated on glass, high-density reaggregate cultures gave rise to approximately 23% hNPCs (βIII-tubulin+/nestin–/vimentin–/GFAP–/NeuN–/MAP2ab+/–), 74% GFAP+ hNPCs (GFAP+/βIII-tubulin+) and 3% O4+/GFAP– oligodendrocyte precursor cells (OPC, compact nuclei, thin-processes) when classified by antigenic protein expression (Figure 5D–H). Morphologically, βIII-tubulin+ labeled hNPCs either had compact nuclei or were asymmetrically bipolar with single long processes (Figure 5E) or were broad with diffuse nuclei and numerous shorter processes (Figure 5E). These data suggest that the cellular constituents of neurosphere reaggregates can give rise to multiple neuronal and glial phenotypes when plated in LIF-containing culture media, similar to the cell types generated from neurosphere cultures of fetal tissue (Weible and Chan-Ling, 2007).When cultured in media supplemented with LIF, both neurospheres and reaggregate neurospheres continued to proliferate and expand as floating spheroid aggregates. We investigated the cellular composition of cryosectioned neurospheres compared with reaggregated neurospheres by multimarker immunocytochemistry after the spheres reached approximately 400 μm in diameter. Both neurospheres (Figure 6A–B, merged in 6C) and reaggregate neurospheres (Figure 6E–F, merged in 6G; 6I–J merged in 6K) were heterogeneous with regard to cellular composition. There was, however, a fundamental difference in cellular organization. While the cells within neurospheres (low-density) appeared stochastic in location and homogeneous in overall composition (Figure 6C, zoomed-in 6D), the reaggregate neurospheres (high-density) were stratified and characterized by an outer layer of nestin–/vimentin– (Figure 6G, zoomed-in 5H); βIII-tubulin+/GFAP– (Figure 6K, zoomed-in 6L); with a few MAP2ab+ cells (neuronal marker) mostly at the surface (Figure 6M–N, merged in 6O; zoomed-in 6P). The halo-staining of βIII-tubulin+ circumventing compact nuclei is classical staining observed in immature neurons and was observed on the sphere surface. hNPCs within the organotypic sphere were also NeuN– (Figure 6Q) but did generate NeuN+ cells (neuronal marker) with compact nuclei or GFAP+ cells (glial marker) with diffuse nuclei when plated in differentiation media (Figure 6R–S, merged in 6T).
Figure 7| Effects of tissue culture condition on organotypic reaggregate neurosphere phenotype.
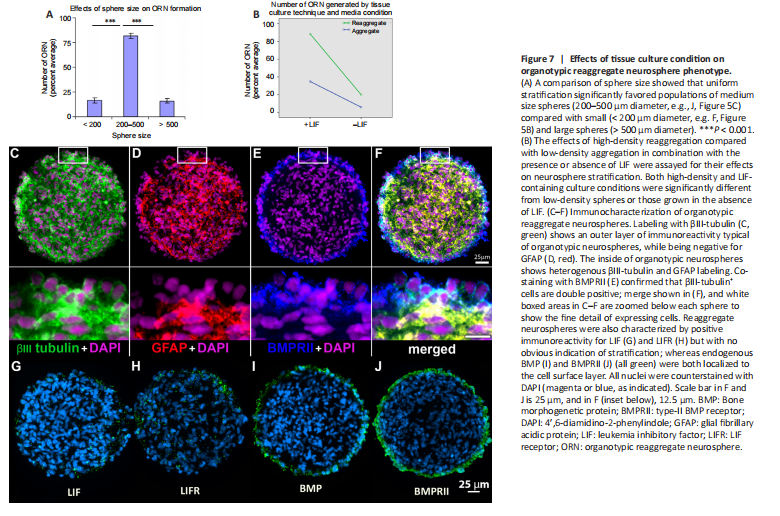
o optimize the culture conditions necessary to generate organotypic reaggregate neurospheres, we first analyzed the effect sphere size had on surface stratification by separating spheres into small, medium, and large. Positive immunostaining of βIII-tubulin+/GFAP–, as in Figure 6I–L, was counted as positive in cryosections and analysis showed percent total organotypic reaggregate neurospheres differed significantly as a function of sphere size (F(2, 57) = 140.98, P < 0.001). Surface stratification significantly increased when spheres were of medium size (200–500 μm diameter) when compared with either small spheres (< 200 μm diameter) or large spheres (> 500 μm diameter; Figure 7A). In subsequent experiments, we selected medium-sized spheres using a standard 200 μl pipette tip with an internal bore of 500 μm.
We next assayed high-density (2.5 × 106 cells/mL) and low-density (2.5 × 104 cells/mL) spheres for surface stratification with and without LIF present in the media (Figure 7B). In this analysis, we also tested if there was an interaction between culture density and LIF using two-way ANOVA. We found a statistically significant effect of LIF on organotypic reaggregate neurosphere formation (F(1,116) = 302.5, P < 0.001) with increased stratification for LIF-containing media (statistical mean, M = 61.6) compared with basal media alone (M = 12.9) with a strong effect size (ηp2 = 0.723, 95% CI: 0.64–0.78). We also found a statistically significant effect of reaggregation on stratification (F(1,116) = 145.8, P < 0.001) where high-density passage increased organotypic reaggregate neurospheres (M = 54.16) compared with low-density (M = 20.40) through the effect was moderate (ηp2 = 0.557, 95% CI: 0.38–0.71). While there was a statistically significant interaction between media condition and tissue culture technique on stratification (F(1,116) = 49.6, P < 0.001) this was a relatively weak effect (ηp2 = 0.299, 95% CI: 0.17–0.46) and most likely inconsequential. Approximately 85–90% of the reaggregates generated in LIF-containing media were stratified with an outer layer of βIII-tubulin+/GFAP– cells (Figure 7B). Therefore, in subsequent experiments, we employed a protocol of reaggregation and expansion in LIF-containing media.
We also characterized the reaggregate spheres for the expression of LIFR, BMP, and BMPR. Positive immunoreactivity was found for both LIF and LIFR stochastically throughout the sphere. Given that these spheres were cultured in LIF-containing media, the observed immunostaining for LIF may have been LIF that was derived from culture media. No apparent stratification to the staining pattern was observed for either LIF or its receptor (Figure 7G and H). Organotypic reaggregate neurospheres were immunopositive for endogenous BMP4 and its receptor, BMPRII. Both were observed to be localized on the surface layer of the spheres (Figure 7I and J). Multi-marker immunostaining of BMPRII together with βIII-tubulin and GFAP (Figure 7C–E) showed co-labeling of BMPRII (Figure 7E) with the βIII-tubulin+/GFAP– hNPCs on the surface (Figure 7F).
Figure 8|Characterization of fluorescence-assisted cell sorting-sorted BMPRII+ hNPCs.

Reaggregate cultures were dissociated, immunostained for a cell surface epitope of BMPRII recognized and sorted as live cells by FACS. Sorted cells were washed, plated on glass in culture media and characterized using antibodies against MAP2ab, GFAP, and O4 (Figure 8C–E, merged in A, brightfield in B). Compared with control (unlabeled) we found approximately a 3.5-fold significant increase in the number of neurons detected using MAP2ab+/βIII-tubulin+ (t(16) = 12.30, P < 0.001) as the assessment criteria of the sorted cells, to a purity of about 83% versus non-sorted control cells (~24%). Figure 8F is a representative histogram of fluorescence intensity versus cell number of a positive single-label FACS sort for BMPRII+ cells (green) compared with the unlabeled control (red). Sorted cells of the collected fraction (~14%) were plated, fixed and the neural phenotypes were quantified (Figure 8G) against the antibodies as indicated in the summary table (Figure 8H).