神经退行性病
-
Figure 1|Characterization of SAGMs.

Our goal was to develop a microcarrier-based platform that 1) is suitable for mDAP adhesion; 2) supports neuronal differentiation and maturation; 3) allows for long-term storage in liquid nitrogen and generates ready-to-use cell products; and 4) maintains axonal integrity and supports injection without involving cell dissociation and separation steps. Given the small diameter of mDAPs (Additional Figure 1A and B), we prepared porous SAGMs, speculating that the high ratio of surface area to volume would enable high-density cell culture and efficient cell production. Scanning electron microscopy analysis showed that the diameter of the SAGMs was 180–300 μm, with an average of 241.2 ± 20.32 μm (n = 150), and the pore size ranged from 5 to 40 μm, with an average of 22.8 ± 5.78 μm (n = 150) (Figure 1A and B). The average Young’s modulus of the SAGMs was 13.65 ± 9.15 kPa, corresponding to lower mechanical stiffness than that of commercial SAGM products (57.23 kPa) (Yan et al., 2020) (Figure 1C). In addition, the SAGMs were dispersed in the culture medium and exhibited a spherical appearance (Figure 1D).
Figure 2|Establishment of SAGM-based mDAP differentiation system.

To assess the effect of SAGM-based mDAP differentiation, day 17 cells were seeded to 2D culture plates or SAGMs. Scanning electron microscopy analysis showed strong attachment of cells and extensive elongation of mDAP axons on SAGMs on day 18 and day 25, respectively (Figure 2B and C). Immunofluorescence assays demonstrated that day 25 cells retained robust expression of the mDAP markers forkhead box protein A2 (FOXA2), LMX1A, OTX2, and EN1 in both the 2D and mDAP beads platforms, whereas no OCT4+ cells were detected. In line with the scanning electron microscopy results, the cells exhibited a typical neuronal morphology, as evidenced by expression of the neuronal marker TUJ1 (Figure 2D). Moreover, the proportions of TUJ1+ and TH+ mDAPs were significantly (TUJ1: P < 0.05; TH: P < 0.001) increased in mDAP beads (TUJ1: 79.8 ± 4.1%; TH: 38.5 ± 1.9%) compared to the 2D control (TUJ1: 74.5 ± 2.4%; TH: 26.1 ± 4.9%). In contrast, the proportion of Ki67+ proliferating cells was markedly lower in the mDAP beads (3.9 ± 1.2% vs. 10.6 ± 0.7%) (Figure 2D and E). Notably, mDAP beads yielded more cells than the 2D control (Figure 2F). In addition, immunofluorescence assays of three batches of mDAP beads demonstrated that the cells retained robust expression of the mDAP markers FOXA2, LMX1A, OTX2, EN1, TH, and TUJ1, showing consistent batch-to-batch quality (Additional Figure 3I and Figure 2D).
Figure 4|Development of the ready-to-use mDAP bead product.
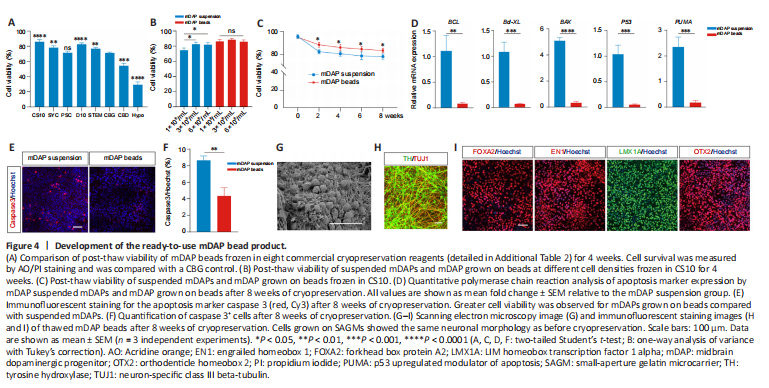
Cryopreservation is essential to facilitate storage of off-the-shelf regenerative medicine products. Thus, we next assessed the post-thaw cell viability and retrieval from mDAP beads frozen in eight commercial cryopreservation reagents. After 4 weeks of cryopreservation, cryovials were thawed using a controlled rate cell-thawing instrument, and cell viability was determined. CS10 (86.9 ± 1.4%) and D10 (83.4 ± 0.7%) yielded high cell viabilities, and CS10 was used in the following experiments (Figure 4A). Compared with suspended mDAPs, the mDAP beads exhibited higher post-thaw cell viability at all cell densities tested (Figure 4B and C). qPCR analysis showed that the mRNA levels of the apoptosis markers BCL, Bcl-XL, BAX, P53, and p53 upregulated modulator of apoptosis (PUMA) (Ming et al., 2006; Follis et al., 2013) in mDAP beads were decreased significantly compared with suspended mDAPs (BCL: P < 0.01; Bcl-XL, BAX, P53, PUMA: P < 0.001) (Figure 4D), which was further supported by immunofluorescence staining for the apoptosis marker caspase-3 (Figure 4E and F). Moreover, the cells on the thawed mDAP beads retained the same phenotype as before freezing (Figure 4G–I). These results suggest that cryopreserved mDAP beads are capable of maintaining cell viability, neuronal morphology, and expression profiles, allowing for the development of ready-to-use mDAP bead products.
Figure 5|Functional characterization of mature mDAP beads in vitro.
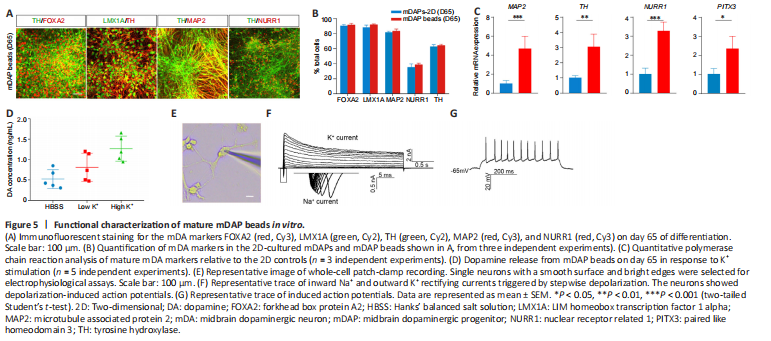
Next, we cultured mDAP beads until day 65 and assessed the expression of markers of mature mDA cells. We found that a large proportion (> 60%) of TH+ cells coexpressed FOXA2, LMX1A, and MAP2. Notably, 38.9% of the cells were NURR1-positive, suggesting differentiation into mature mDAs. In addition, no significant difference was observed between the expression levels of these protein markers in mDAP beads and the 2D control (Figure 5A, B and Additional Figure 4A), while higher mRNA levels of the mature mDA neuron markers MAP2, TH, NURR1, and paired like homeodomain 3 (PITX3) were observed in mDAP beads compared with 2D culture (Figure 5C). Given that dopamine synthesis is critical for the function of mDA neurons, next we measured dopamine release from cells on day 65 in response to stimulation with low (4.7 mM) and high (56 mM) concentrations of KCl by high-performance liquid chromatography. The cells released 0.49 ± 0.12 μg/mL of dopamine in Hanks’ balanced salt solution, 0.75 ± 0.27 μg/mL in low KCl, and 1.41 ± 0.43 μg/mL in high KCl, indicating their capacity to release dopamine in response to K+-induced depolarization (Figure 5D). To characterize the electrophysiological properties of mDAP beads, we performed whole-cell patch-clamp recording on day 65 (Figure 5E). The neurons showed representative traces of inward Na+ and outward K+ rectifying currents triggered by stepwise depolarization, and 60% of the neurons tested showed induced action potentials (n = 15) (Figure 5F and G). Together, these findings demonstrate that mDAP beads can be cultured long term to generate mature mDA neurons.
Figure 6|Enhanced graft survival and reduced immunogenicity.
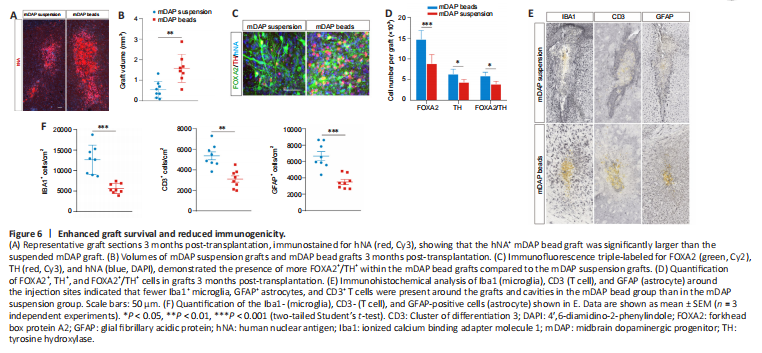
To evaluate the post-transplantation outcomes of mDAP beads in vivo, we transplanted suspended mDAPs or mDAP beads into the striatum of SCID mice. After 3 months, immunofluorescence staining for human nuclear antigen (hNA) showed surviving grafts in both groups. Stereological analysis revealed that the hNA+ graft in the animals that received mDAP beads (1.58 mm3) was significantly (P < 0.01) larger than that in the animals injected with the mDAP suspension (0.54 mm3) (Figure 6A and B). Additionally, more FOXA2+/TH+ neurons were observed within the mDAP bead grafts (5.9 ± 0.9%) compared with the mDAP suspension grafts (3.9 ± 0.6%) (Figure 6C and D). Although the brain is considered to be a relatively immune-privileged organ, intracerebral graft-induced immune rejection remains a major issue for allogenic neural transplantation, which results in reduced neuronal survival (Aron Badin et al., 2019; Kim et al., 2020). We found fewer Iba1+ microglia, GFAP+ astrocytes, and CD3+ T cells around the grafts and cavities in the mDAP bead group than in the mDAP suspension group, indicating reduced immunogenicity of the mDAP beads compared with suspended mDAPs (Figure 6E and F). Overall, our in vivo results verified that the microcarrier-based strategy maintains axonal integrity and improves post-transplantation outcomes.
点击此处查看全文