脑损伤
-
Figure 1|Representative photographs of SAH mice and the expression levels of TSG-6, NLRC4, GSDMD, and GSDMD-N after SAH.
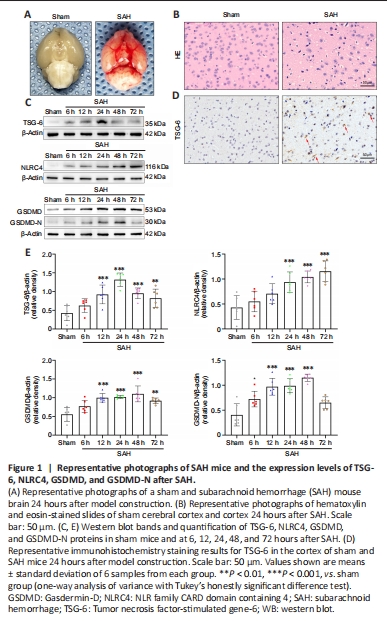
Of the 321 experimental mice, 71 and 250 were allocated to the sham and various treatment groups, respectively. In all experiments, 7 mice were excluded according to the prior exclusion criteria. Overall, mortality in the sham and SAH groups was 0 and 17.28%, respectively. In experiment 1, the corresponding rates were 0% and 16%, respectively. In experiment 2, mortality in the sham, SAH, SAH + vehicle, and SAH + rh-TSG-6 groups was 0%, 16.22%, 18.42%, and 11.43%, respectively. In experiment 3, mortality in the sham, SAH, SAH + scramble siRNA, and SAH + TSG-6 siRNA groups was 0%, 15.38%, 18.52%, and 26.67%, respectively. The mortality rates overall and according to group in the three experiments are presented in Additional Table 1. Representative photographs of a sham and SAH mouse brain are exhibited in Figure 1A. In the SAH mice, SAH was primarily seen along the inferior basal temporal lobe and the circle of Willis, which are the regions that exhibit the most notable molecular biological changes (Park et al., 2004). Hematoxylin and eosin staining showed histological damage in the inferior basal temporal cortex 24 hours after SAH (Figure 1B).
Figure 2| TSG-6 is expressed in astrocytes and neurons after subarachnoid hemorrhage (SAH).
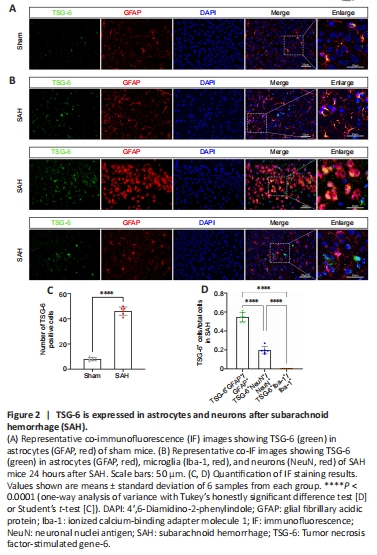
IHC analysis revealed that TSG-6 expression was considerably higher 24 hours after SAH than in the sham group (Figure 1D). Double-labeled IF staining for TSG-6 with GFAP, NeuN, or IBA-1 demonstrated that TSG-6 was predominantly expressed in astrocytes and to a minor extent in neurons of the ipsilateral hemisphere; moreover, the number of TSG-6-positive astrocytes was significantly augmented 24 hours after SAH compared with the sham group (P < 0.0001; Figure 2A–D). Microglia, however, did not show TSG-6 immunostaining (Figure 2B).
Figure 3|rh-TSG-6 administration inhibits NLRC4 inflammasome activation and the expression of its downstream pyroptosis-related proteins after subarachnoid hemorrhage (SAH).

Both IF and IHC staining confirmed that NLRC4 protein level remarkably increased; however, this increase was inhibited by rh-TSG-6 administration 24 hours after SAH (Figure 3A and B). Compared with the sham group, the SAH and SAH + vehicle groups exhibited a remarkable increase in NLRC4 expression, while rh-TSG-6 treatment significantly downregulated NLRC4 expression (P < 0.01; Figure 3E and F).
GSDMD expression in astrocytes was significantly higher after SAH. rh-TSG-6 administration, however, reduced GSDMD expression (Figure 3C and D). rh-TSG-6 reduced the expression of caspase-1 in astrocytes after SAH (Additional Figure 3). Consistent with the IF and IHC staining results, similar alterations in the expression levels of GSDMD and GSDMD-N were confirmed by WB analysis (Figure 3E and F). Moreover, the WB results demonstrated that the levels of cleaved caspase-1, IL-18, and IL-1β were distinctly higher in the SAH and SAH + vehicle groups than in the sham group, whereas rh-TSG-6 treatment significantly restrained the expression of these proteins (cleaved caspase-1, P < 0.0001; IL-18, P < 0.01; IL-1β, P < 0.001; Figure 3E and F). Consistent with the WB results, ELISA identified that the increases in IL-18 and IL-1β levels caused by SAH were reversed by rh-TSG-6 treatment (Figure 3G). TEM showed that the pores of the astrocyte membrane were correspondingly reduced after rh-TSG-6 treatment (Figure 4C).
Figure 4|rh-TSG-6 administration alleviates short-term neurological deficits, brain edema, cell apoptosis, and astrocyte pyroptosis after subarachnoid hemorrhage (SAH).
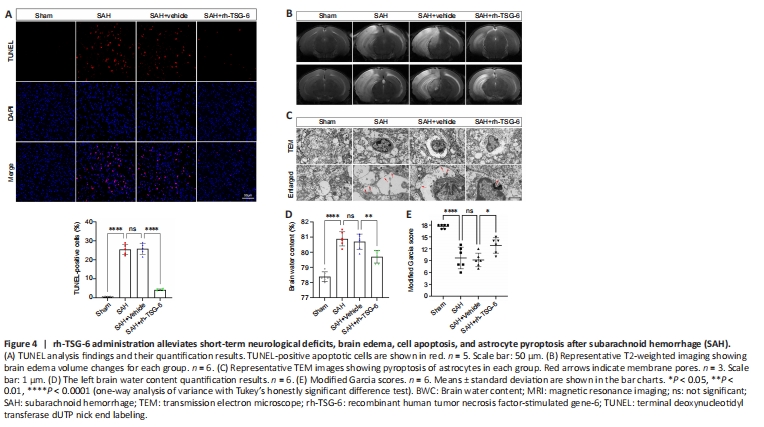
TUNEL staining revealed that the number of apoptotic cells was remarkably higher in the SAH and SAH + vehicle groups than the sham group. rh-TSG-6 administration significantly lowered the number of apoptotic cells after SAH (P < 0.0001; Figure 4A). FJC staining showed that neuronal degeneration was enhanced after SAH and rh-TSG-6 treatment considerably decreased the number of cells stained with FJC (Additional Figure 4).
Left hemisphere BWC was higher in the SAH and SAH + vehicle groups than the sham group (P < 0.0001; Figure 4D). BWC remarkably decreased after rh-TSG-6 treatment (P < 0.01; Figure 4D). Additionally, mice in the SAH group exhibited considerable cerebral edema in the left hemisphere on MRI; however, rh-TSG-6 administration greatly mitigated this (Figure 4B). The modified Garcia test revealed that treatment with rh-TSG-6 ameliorated neurological deficits in SAH mice (Figure 4E).
Figure 5| siRNA downregulates the expression of TSG-6 24 hours after SAH.
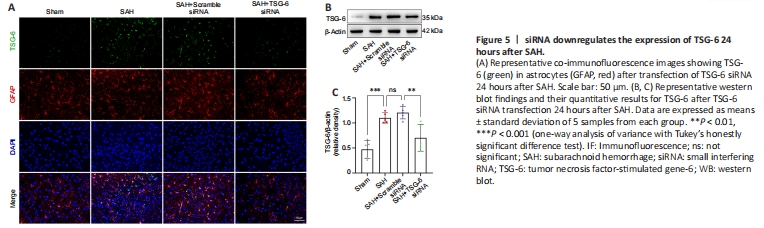
Figure 6|Knockdown of TSG-6 amplified NLRC4 inflammasome activation and the expression level of its downstream pyroptosis-related proteins after SAH.
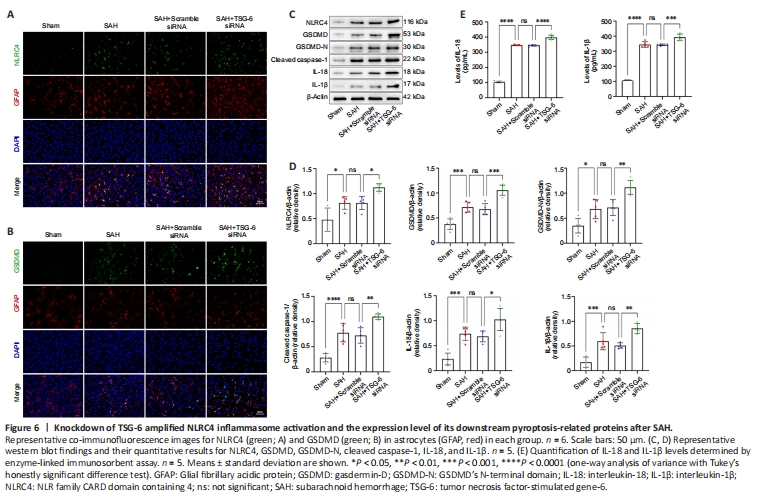
The TSG-6 gene was silenced using an siRNA and successful knockdown of the TSG-6 gene was confirmed by IF and WB (Figure 5A–C). IF confirmed that the NLRC4 protein level was further elevated after TSG-6 knockdown relative to that in the SAH and SAH + NC siRNA groups 24 hours after SAH (Figure 6A). IF also confirmed that the level of GSDMD, a primary marker for detecting pyroptosis, was considerably elevated in the TSG-6 siRNA group relative to that in the SAH and SAH + NC siRNA groups (Figure 6B). Likewise, IHC staining showed identical variations in the expression levels of NLRC4 and GSDMD in accordance with the results of IF staining (Additional Figure 5). Consistent with the IF and IHC staining results, similar alterations in the expression levels of NLRC4 and GSDMD were confirmed by WB (Figure 6C). WB revealed that TSG-6 siRNA further increased the levels of GSDMD-N, cleaved caspase-1, IL-18, and IL-1β after SAH (Figure 6C and D). ELISA also demonstrated similar alterations in the expression levels of IL-1β and IL-18 in the various SAH groups (Figure 6E).
Figure 7| Knockdown of TSG-6 worsened cell apoptosis, neuronal injury, neurological deficits, and brain edema after SAH.
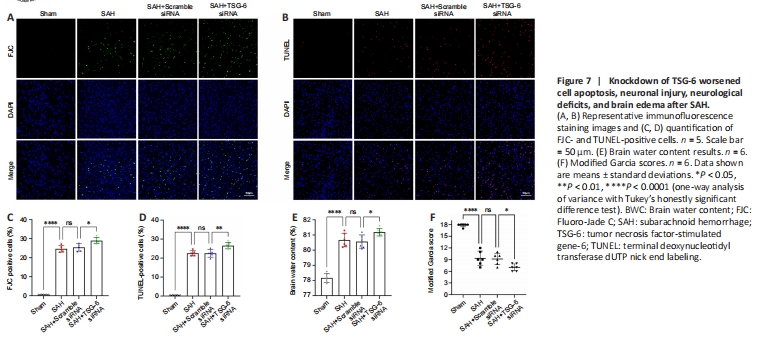
IF staining confirmed that the numbers of FJC-positive and TUNEL-positive cells were higher in the TSG-6 siRNA group than the SAH and SAH + NC siRNA groups (P < 0.05 for FJC, P < 0.01 for TUNEL; Figure 7A–D). TSG-6 knockdown mice showed more severe left cerebral hemisphere edema than mice in the SAH and SAH + NC siRNA groups (P < 0.05; Figure 7E). Moreover, TSG-6 siRNA treatment worsened neurological deficits (Figure 7F).