NRR:南方医科大学段传志团队报道TSG-6可减轻蛛网膜下腔出血后的细胞焦亡
撰文:丁明祥 魏博洋
蛛网膜下腔出血(SAH)是一种非常凶险的脑卒中亚型,主要由颅内动脉瘤破裂引起,具有较高的发病率和死亡率[1]。超过50%的幸存者仍然存在神经功能障碍。根据近期研究,SAH后早期脑损伤(EBI)是临床预后不良的重要因素[2]。细胞焦亡是近些年发现的一种炎症小体介导的依赖天冬氨酸特异性的半胱氨酸蛋白水解酶(caspase-1)的程序性细胞死亡过程[3]。目前已经确定细胞焦亡是SAH后EBI的重要机制。然而,目前仍缺乏对于SAH后细胞焦亡的有效治疗方案。肿瘤坏死因子刺激基因6 (TSG-6)是一种相对较小的透明质酸结合糖蛋白,可以参与多种炎症性疾病的病理过程并发挥抗炎作用,属于一个保护性蛋白。近来一些研究报告结果表明TSG-6可以减轻脑损伤[4,5]。作者团队之前的研究发现,SAH后给予TSG-6可通过抑制炎症反应和氧化应激显著缓解EBI[5,6]。然而,TSG-6对SAH后EBI的缓解作用是否包含抑制细胞焦亡和任何其他潜在机制尚不明确。
近期,南方医科大学段传志团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“TSG-6 ameliorates early brain injury after subarachnoid hemorrhage by suppressing NLRC4 inflammasome-mediated astrocyte pyroptosis”的研究论文。研究发现,外源性TSG-6的治疗可以明显减轻蛛网膜下腔出血后早期脑损伤中星形胶质细胞焦亡,并可改善小鼠脑水肿程度及神经功能缺损。进一步研究证实,TSG-6是通过抑制NLRC4炎症小体通路缓解星形胶质细胞焦亡。该文带来的启示:TSG-6是减轻蛛网膜下腔出血后细胞焦亡的潜在药物,可为改善蛛网膜下腔出血预后提供新的治疗方式。
该研究首先通过免疫组化实验检测了TSG-6蛋白在SAH后24小时的表达情况。实验结果表明在SAH后24小时,TSG-6蛋白的阳性表达相对于sham组明显升高(图1)。
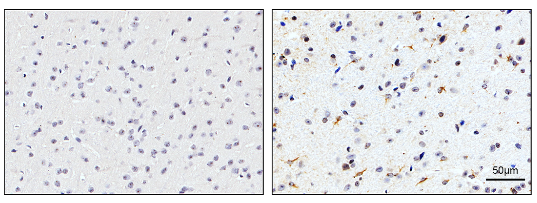
图1:Sham组和SAH后24小时组脑皮层组织中TSG-6的免疫组化染色结果(Ding et al., 2024)
其次,通过Western blot实验证实,在SAH后TSG-6蛋白的阳性表达水平较sham组明显升高,并在SAH后24小时表达达峰(图2)。免疫荧光双染色实验证实TSG-6蛋白主要在星形胶质细胞中表达,在神经元有少量表达,在小胶质细胞中未见到表达(图3)。研究中采用Western blot检测方法分别测定sham组和SAH组在出血后的6,12,24,48,72h大脑颞底皮层组织中相关蛋白的表达变化。结果显示,与sham组相比NLRC4,GSDMD,GSDMD-N蛋白的表达在SAH后均明显升高。
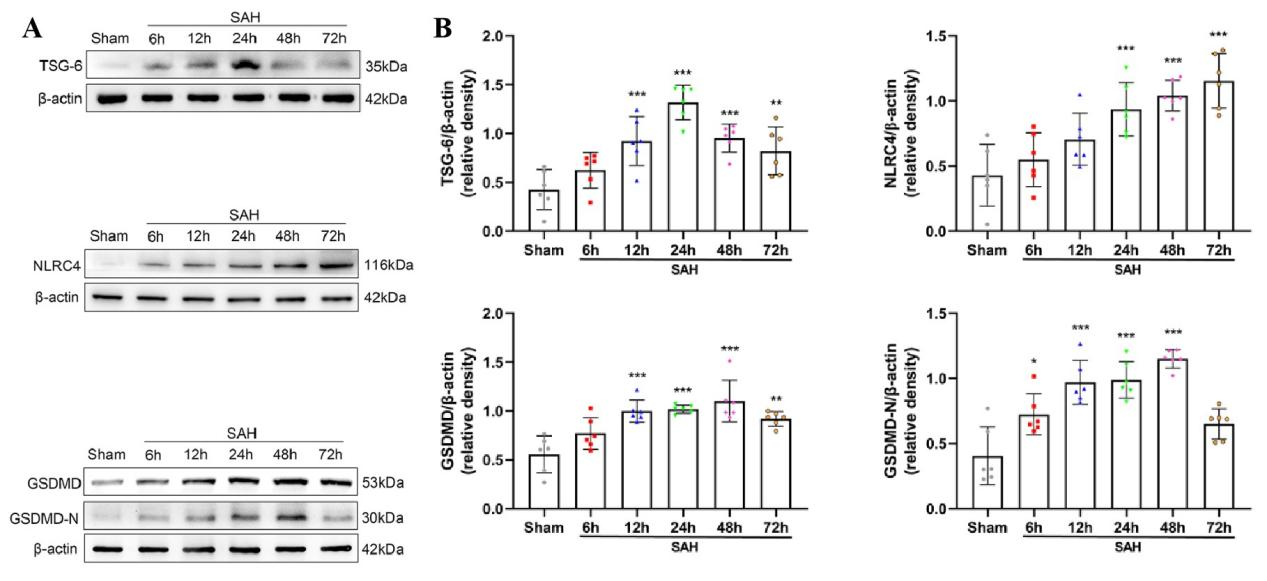
图2: SAH后TSG-6,NLRC4,GSDMD,GSDMD-N蛋白的表达水平明显升高(Ding et al., 2024)
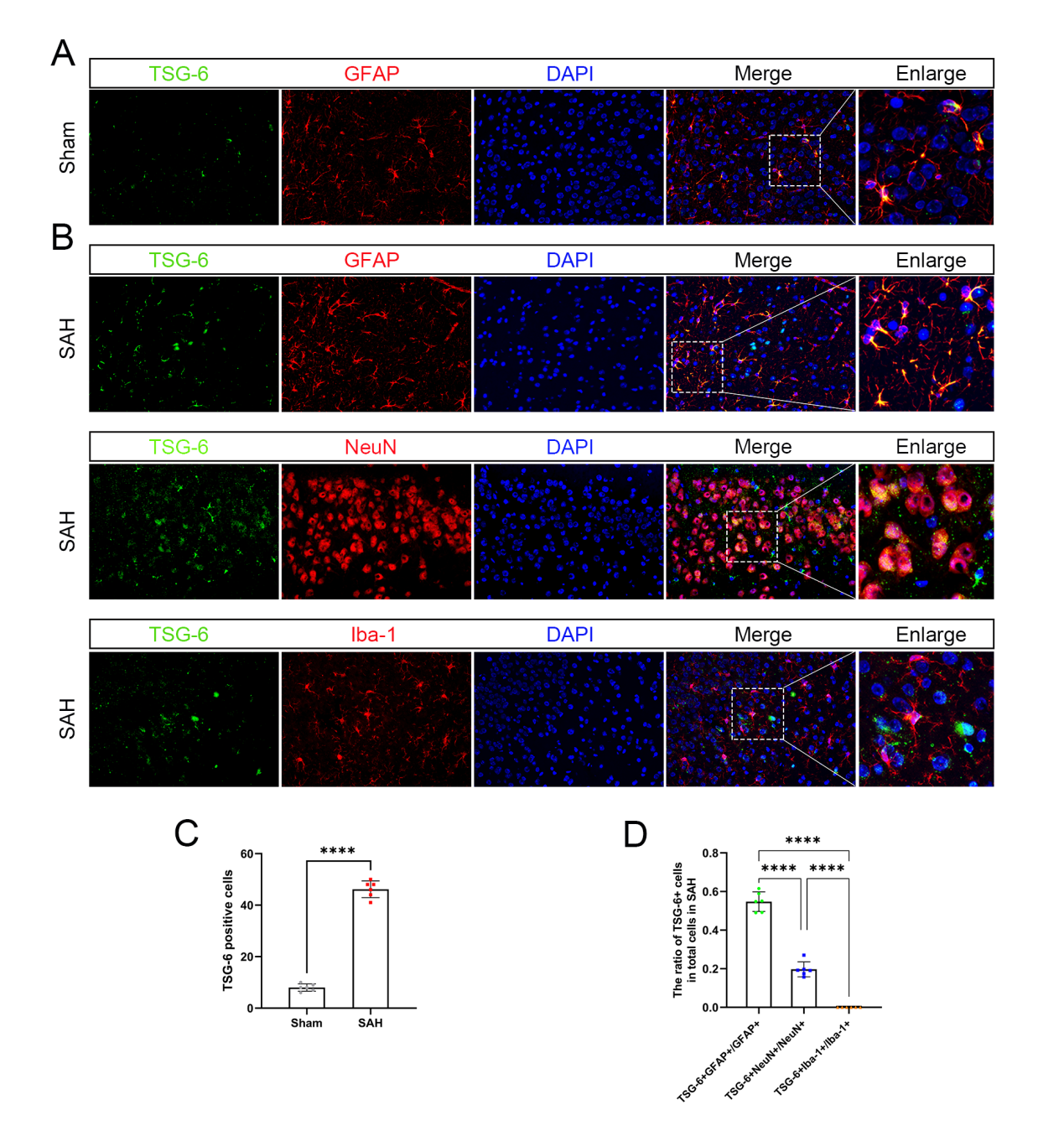
图3:TSG-6蛋白在脑皮层组织中的表达情况及细胞定位(Ding et al., 2024)
在小鼠SAH模型手术实施后半小时给予外源性TSG-6脑室注射,并在SAH模型手术实施后24小时,对小鼠脑水肿进行测定并采用改良Garcia评分对小鼠神经功能进行评测。与sham组相比,SAH组小鼠脑水肿程度明显增加,神经功能评分明显下降。当给予外源性TSG-6脑室注射后,相比于SAH + vehicle组小鼠,SAH + rh TSG-6组小鼠脑水肿程度及神经功能缺损情况明显改善(图4A, B)。
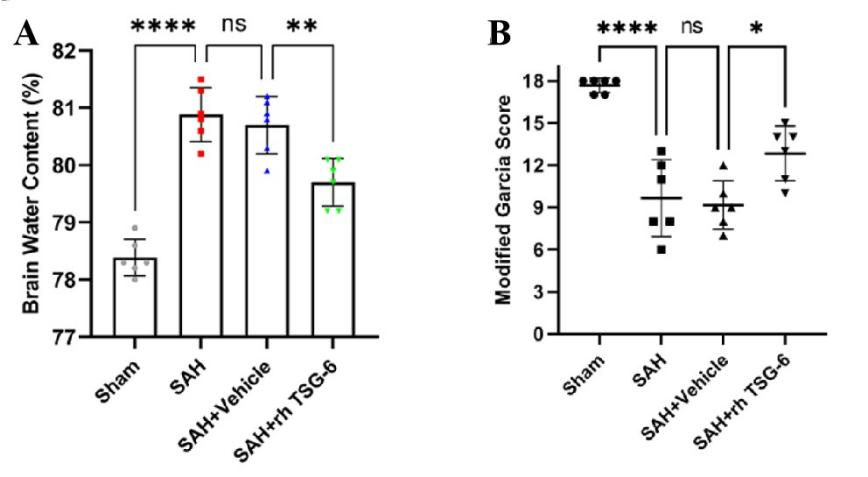
图4:外源性TSG-6注射可改善小鼠SAH后脑水肿及神经功能缺损(Ding et al., 2024)
为进一步检测TSG-6对SAH后脑水肿的作用,作者实施了小动物核磁共振检查。结果表明:与sham组相比,SAH组和SAH + vehicle组小鼠脑水肿程度明显严重。当给予外源性TSG-6脑室注射后,相比于SAH组和SAH + vehicle组小鼠,SAH + rh TSG-6组小鼠仍存在脑水肿情况,但脑水肿严重程度明显改善(图5)。
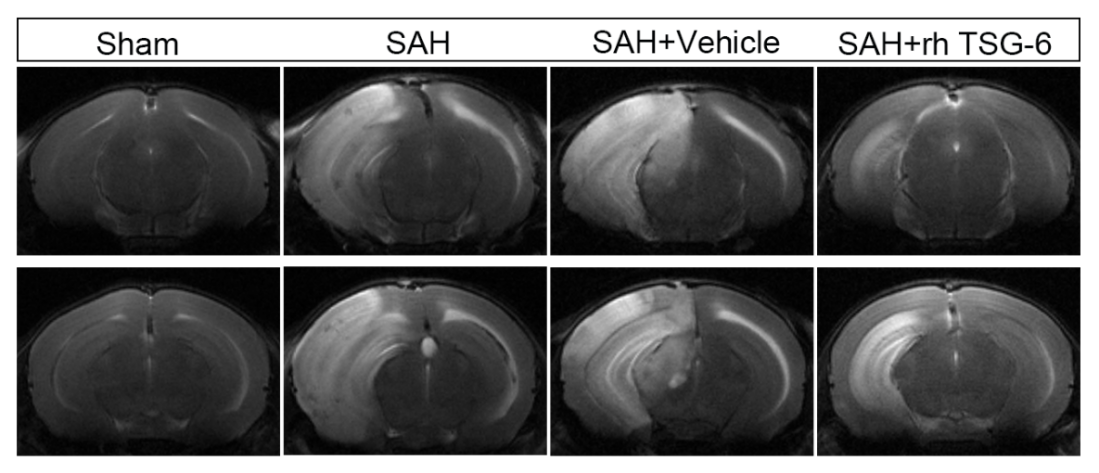
图5:外源性TSG-6注射可改善小鼠SAH后脑水肿及神经功能缺损(Ding et al., 2024)
采用Western blot检测方法分别测定sham组、SAH组、SAH + vehicle组和SAH + rh TSG-6组在SAH后的24小时,大脑颞底皮层组织中相关蛋白的表达变化。与sham组相比,SAH组和SAH + vehicle组小鼠脑颞底皮层组织中NLRC4,GSDMD,GSDMD-N,cleaved caspase-1,IL-18和IL-1β的表达量明显升高。但当给予外源性TSG-6脑室注射治疗后,相比于SAH + vehicle组小鼠,SAH + rh TSG-6组小鼠脑颞底皮层组织中以上蛋白的表达均有明显下降(图6)。
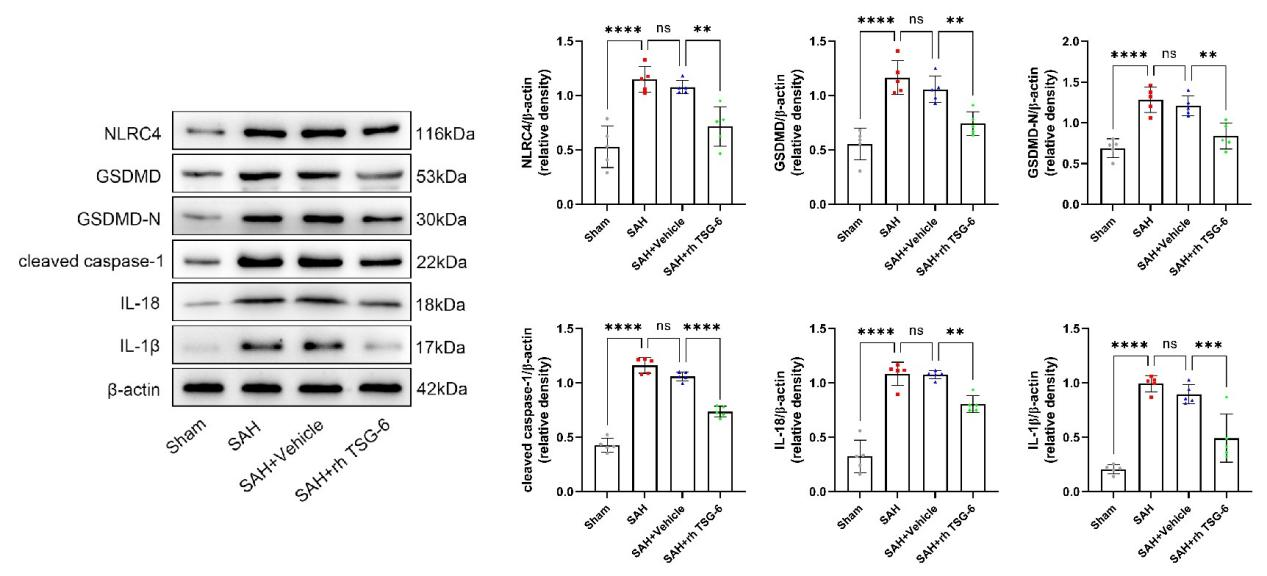
图6:每组NLRC4,GSDMD,GSDMD-N,cleaved caspase-1,IL-18和IL-1β的典型Western blot图像及其定量分析(Ding et al., 2024)
采用免疫组织化学检测方法测定大脑颞底皮层组织中NLRC4、GSDMD蛋白的表达变化。TSG-6可明显降低蛛网膜下腔出血后NLRC4以及GSDMD的表达(图7)。

图7: 外源性TSG-6注射可以抑制小鼠SAH后NLRC4和GSDMD的表达(Ding et al., 2024)
采用TUNEL染色试剂盒分别测定sham组、SAH组、SAH + vehicle组和SAH + rh TSG-6组在SAH后24小时,大脑颞底皮层组织中细胞凋亡情况。与sham组相比,SAH组和SAH + vehicle组小鼠脑颞底皮层组织中细胞凋亡数目显著增多。但当给予外源性TSG-6脑室注射治疗后,相比于SAH + vehicle组小鼠,SAH + rh TSG-6组小鼠脑颞底皮层组织中细胞凋亡数目明显降低(图8)。

图8:SAH后24小时各组大脑皮层的TUNEL染色结果及定量分析(Ding et al., 2024)
在SAH模型手术实施后24小时取材行脑组织透射电子显微镜检。sham组中正常星形胶质细胞形态饱满,胞膜完整。SAH组、SAH + vehicle组和SAH + rh TSG-6组中可见星形胶质细胞焦亡表现,胞膜穿孔不连续(图9)。
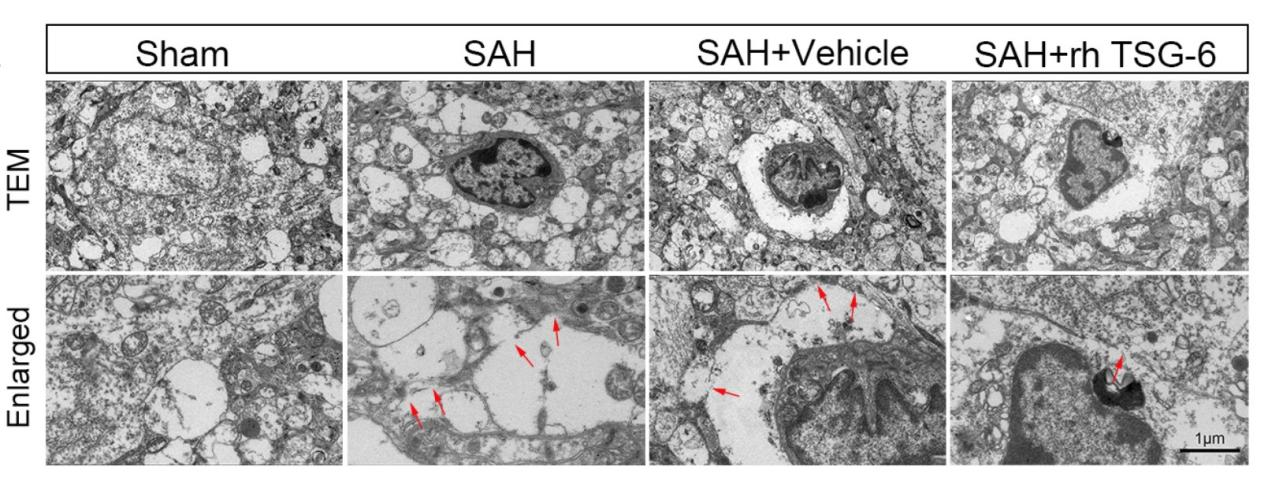
图9:各组小鼠SAH后24h脑组织中典型星形胶质细胞焦亡的透射电子显微镜图片(Ding et al., 2024)
在小鼠SAH模型手术实施前48小时给予TSG-6 siRNA脑室注射,在SAH模型手术实施后24小时取材。采用干湿比重法评估敲减TSG-6表达后小鼠脑水肿情况。与sham组相比,SAH组小鼠脑水肿程度明显严重。当给予干扰RNA敲减TSG-6表达后,相比于SAH + scramble siRNA组小鼠,SAH +TSG-6 siRNA组小鼠脑水肿严重程度加重(图10A)。采用改良Garcia评分对小鼠神经功能进行评测。与sham组相比,SAH组的神经功能评分明显下降。当给予干扰RNA敲减TSG-6表达后,相比于SAH + scramble siRNA组小鼠,SAH + TSG-6 siRNA组小鼠神经功能缺失情况进一步加重,评分更低(图10B)。
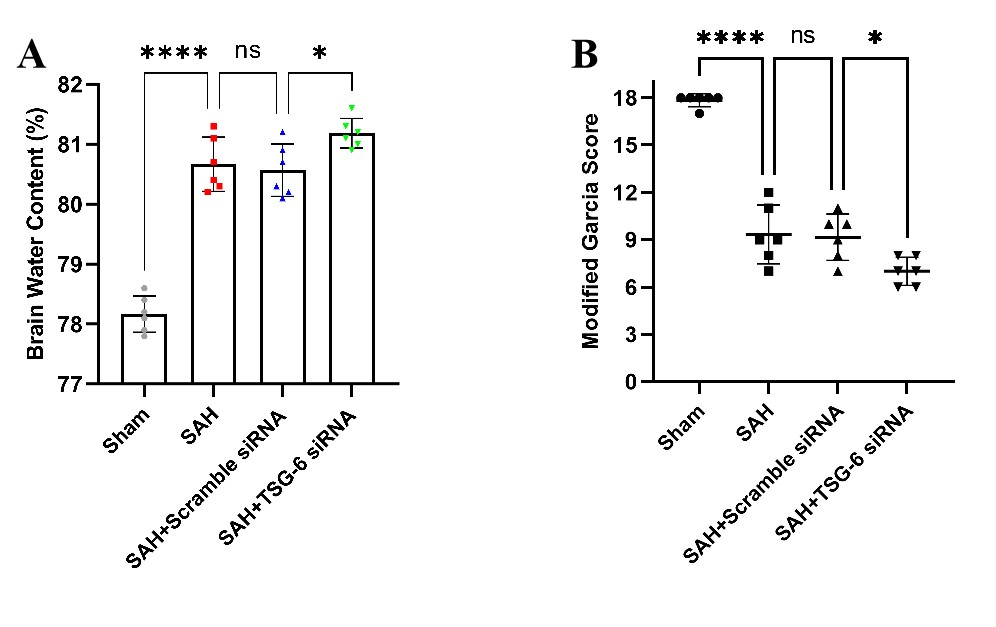
图10:TSG-6 siRNA注射加剧了小鼠SAH后脑水肿及神经功能(Ding et al., 2024)
研究者继续探究了TSG-6 siRNA对大脑颞底皮层组织中相关蛋白的表达变化。与sham组相比,SAH组和SAH + scramble siRNA组小鼠脑颞底皮层组织中NLRC4、GSDMD、GSDMD-N、cleaved caspase-1、IL-18和IL-1β的表达量明显升高。但当给予TSG-6 siRNA脑室注射后,相比于SAH + scramble siRNA组小鼠,SAH + TSG-6 siRNA组小鼠脑颞底皮层组织中以上蛋白的表达进一步升高(图11)。
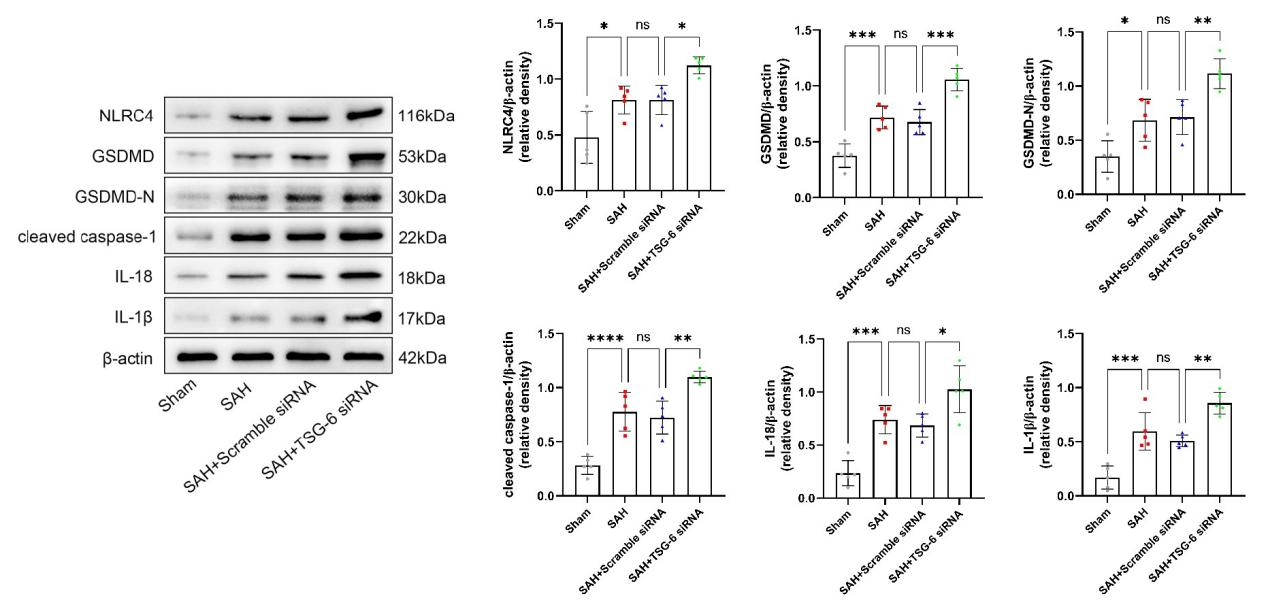
图11:每组NLRC4,GSDMD,GSDMD-N,cleaved caspase-1,IL-18和IL-1β的典型Western blot图像及其定量分析(Ding et al., 2024)
采用FJC染色试剂盒探测了TSG-6 siRNA对大脑颞底皮层组织中神经元坏死情况。与sham组相比,SAH组和SAH + scramble siRNA组小鼠脑颞底皮层组织中神经元变性数目显著增多。当给予TSG-6 siRNA脑室注射后,相比于SAH + scramble siRNA组小鼠,SAH + TSG-6 siRNA组小鼠脑颞底皮层组织中神经元变性数目进一步增加(图12)。
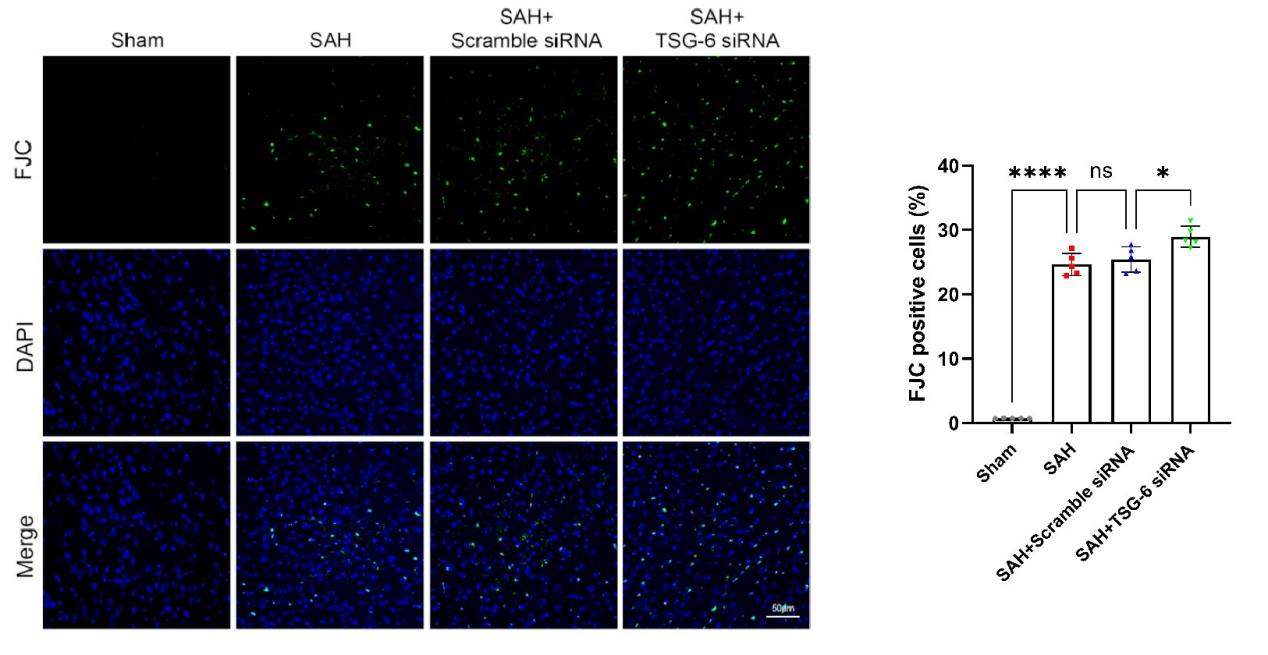
图12:SAH后24小时各组大脑皮层的FJC染色结果及定量分析(Ding et al., 2024)
作者分别采用脑室内注射rh-TSG-6和siRNA的方式上调以及下调TSG-6的表达。给予外源性rh-TSG-6脑室注射后,相关实验结果显示GSDMD等细胞焦亡相关分子的表达明显下降,并伴随着神经元退变、小鼠脑水肿以及神经功能缺失的改善。但当通过siRNA下调小鼠脑组织中TSG-6的表达后,结果显示之前由rh-TSG-6带来的改善细胞焦亡的作用被削弱,神经元退变、小鼠脑水肿程度及神经功能缺失的程度加重。研究中,免疫荧光双染色实验证实上调和下调TSG-6的表达,可以引发细胞焦亡效应蛋白GSDMD在星形胶质细胞内相对应的表达变化。因此,证实TSG-6可以通过抑制星形胶质细胞的焦亡缓解小鼠SAH诱发的早期脑损伤。
在作者的研究中,给予外源性TSG-6脑室注射后,小鼠脑组织中GSDMD和GSDMD-N的表达明显下调,证明TSG-6可以缓解SAH后小鼠脑组织中的细胞焦亡发生程度。给予外源性TSG-6注射的同时伴随着SAH后小鼠脑组织水肿程度以及神经功能缺损的改善。证实TSG-6可以通过改善细胞焦亡缓解小鼠SAH后EBI。此项研究将为TSG-6治疗蛛网膜下腔出血后早期脑损伤中细胞焦亡提供了基础支持。
当然该研究也存在部分局限性,作者并未对蛛网膜下腔出血3天之后的小鼠进行研究,以观察TSG-6对蛛网膜下腔出血后其他时间点的抗细胞焦亡作用,也未探测TSG-6对小鼠蛛网膜下腔出血后远期神经功能的作用。作者只使用了雄性小鼠进行实验,并未探测TSG-6对雌性小鼠蛛网膜下腔出血后的作用,为避免性别差异带来的偏倚,未来应考虑将雌性小鼠同时纳入研究。研究中TSG-6使用了侧脑室注射的递送方式,并未探讨其他简便易行的给药方式,比如皮下注射,静脉注射等,这为未来的临床治疗带来了不确定性。
原文链接:https://doi.org/10.4103/1673-5374.385311
参考文献
[1] Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(10069):655-666.
[2] Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10(4):349-356.
[3] Yu P, Zhang X, Liu N, et al. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128.
[4] Oh JY, Roddy GW, Choi H, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107(39):16875-16880.
[5] Wisniewski HG, Snitkin ES, Mindrescu C, et al. TSG-6 protein binding to glycosaminoglycans: formation of stable complexes with hyaluronan and binding to chondroitin sulfates. J Biol Chem. 2005;280(15):14476-14484.
[6] Li R, Liu W, Yin J, et al. TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J Neuroinflammation. 2018;15(1):231.
第一作者:丁明祥,金磊,魏博洋
通讯作者:李西锋,段传志
通讯作者介绍:段传志
二级教授、主任医师、博士生(后)导师;
任南方医科大学珠江医院脑血管病中心主任;
广东省颅脑外科医疗质量控制中心副主任;
广东省介入放射质量控制中心副主任。
国家卫计委出血性卒中介入治疗专业委员会副主任委员;
国际血管联盟中国分部脑血管病委员会副主任委员;
任广东省医师协会神经介入医师分会主任委员;
广东省医师协会神经外科分会副主任委员;
广东医学会介入医学分会副主任委员;
广东省介入放射医师协会副主任委员;
中国医师协会神经介入专家委员会常委;
中国卒中学会神经介入分会常委。
该实验依托和承担单位是南方医科大学珠江医院,珠江医院神经外科是国家临床重点专科。本科建有神经外科专用的广东省重点实验室“脑功能修复与再生实验室”,具备各种实验仪器,设备先进,为本课题提供完备的硬件支持。#br#
基金支持:国家自然科学基金(基金号:81974178)
第一作者丁明祥照片:
 #br#
#br#












 #br#
#br#