脑损伤
-
Figure 1|Mortality and behavioral disorders induced by ischemia in a mouse model were rescued by mitochondrial transplantation.
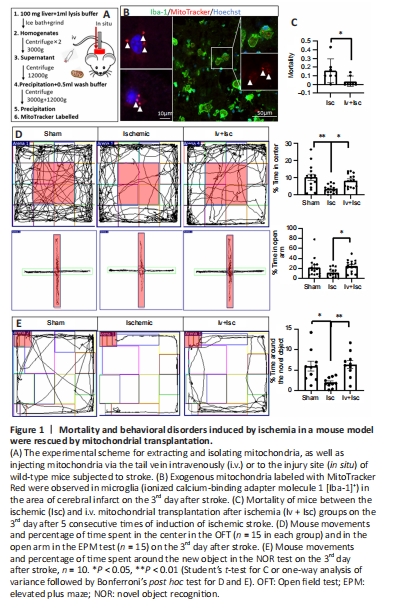
Intravenous mitochondrial transplantation has been shown to effectively alleviate the effect of ischemia-reperfusion injury in mice (Nakamura et al., 2020b). Therefore, we first sought to determine the effect of i.v. mitochondrial transplantation on mortality and behavior in WT mice. We injected freshly isolated mitochondria into the tail vein of mice subjected to focal ischemic stroke and observed the resulting neuroprotective effects (Figure 1A). Firstly, exogenous mitochondria pre-labeled with MitoTracker Red CMXRos probes were clearly seen in microglia in the cortical infarct area (Figure 1B). Furthermore, the mortality rate on the 3rd day after stroke was significantly lower in the Iv + Isc group (P = 0.0478) compared with ischemic group (Figure 1C). The OFT and EPM test were performed three days after stroke to examine anxiety-like behavior, while the NOR test was used to evaluate memory (Sawangjit et al., 2018; Sun et al., 2020b; Liu et al., 2021). As shown in Figure 1D, in comparison with the mice in the Sham group, the mice that had experienced ischemic stroke spent significantly less time in the center of the open field (P = 0.001), indicating increased anxious behavior. In the NOR test, after acclimating to the environment and objects in the chamber, the mice in the ischemic group spent significantly less time around the new object (P = 0.014) than those in the sham group (Figure 1E). Exogenous mitochondrial transplantation i.v. markedly rescued the anxious behavior and impaired memory induced by stroke (P = 0.022, 0.031, and 0.006, respectively; Figure 1D and E). Next, we sought to determine the mechanism(s) responsible for these effects.
Figure 2|Intravenous mitochondrial transplantation reduced cortical infarct area, inhibited pyroptosis, and promoted neurogenesis.
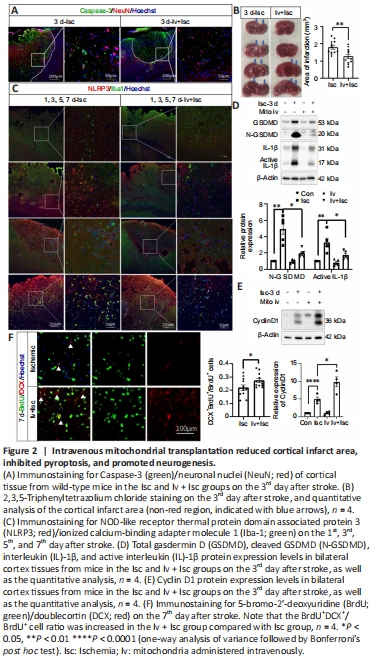
The infarct area in the animals’ brains was determined by Caspase-3/NeuN and TTC staining 72 hours after stroke. Mitochondrial transplantation significantly reduced infarct area compared with saline-treated controls (P < 0.01; Figure 2A and B). On the 1st, 3rd, 5th, and 7th day after stroke, expression of the inflammasome NLRP3 protein was upregulated, and this effect was suppressed by mitochondrial transplantation (Figure 2C). Furthermore, the expression levels of pyroptosis signaling pathway components, including total GSDMD, N-GSDMD, IL-1β, and active IL-1β, were significantly increased in the ischemic cortex (Isc) compared with the contralateral cortex (Con), and this effect was significantly suppressed by transplanting mitochondria i.v. (Iv + Isc) (P < 0.05; Figure 2D). It has been shown that overexpressing cyclin D1 in neural stem cells (NSCs) increases both cell number and cell cycle activity to promote neurogenesis in adult mice (Berdugo-Vega et al., 2020). In this study, we found that cyclin D1 expression increased after ischemia, and its expression level was further elevated by administering mitochondria (Figure 2E). Additionally, the BrdU+DCX+/BrdU+ cell ratio was increased by transplanting mitochondria i.v. on the 7th day after stroke, suggesting that neurogenesis in the ischemic cortex was promoted by transplanting mitochondria (Figure 2F), consistent with a previous study (Zhang et al., 2019). Thus, reducing the infarct area, inhibiting pyroptosis, and promoting neurogenesis may contribute to the amelioration in mortality, anxiety-like behavior, and impaired memory observed in mice subjected to ischemic stroke, as described above.
Figure 4|Mitochondrial transplantation induced upregulation of S100A9, crucial link between neurogenesis and pyroptosis.

RNA-seq (https://www.ncbi.nlm.nih.gov/sra/PRJNA889464), KEGG, and GO analyses showed that the DEGs between the control and ischemic cortex are involved in the PI3K/Akt, NOD-like receptor, MAPK, calcium, and apoptosis signaling pathways, among others (Figure 3A and Additional Figure 1). The DEGs between the Iv + Isc and ischemic groups were involved in Wnt, transcriptional misregulation in cancer, MAPK, endocytosis, calcium, and axon guidance signaling pathways, among others (Figure 3B). Similarly, the DEGs (q < 0.05) between the In situ + Isc and ischemic groups were significantly enriched in the Wnt, transcriptional misregulation in cancer, MAPK, and endocytosis pathways (Figure 3C). Interestingly, 69 overlapping genes from the Iv + Isc vs. Isc DEGs and the In situ + Isc vs. Isc DEGs were involved in pyroptosis, such as Bax, Caspase-3, IL-18, and GSDMD (q < 0.05; Figure 3D), while 335 overlapping mRNA were related to neurogenesis. STAT3, Nestin, cyclin D1 (CCND1), JUN, brain-derived neurotrophic factor, MYC, Wnt3, Wnt2, and Wnt2β, whose mRNA levels were upregulated by transplanting mitochondria either i.v. or in situ, indicated activated neurogenesis (Figure 3E). Thus, the effects of mitochondrial transplantation on cortical pyroptosis and neurogenesis as determined by RNA-seq correlated well with the histological results described above (Figure 2C–F).
Figure 5|S100A9 mediated the beneficial effects of mitochondrial transplantation on pyroptosis and neurogenesis.
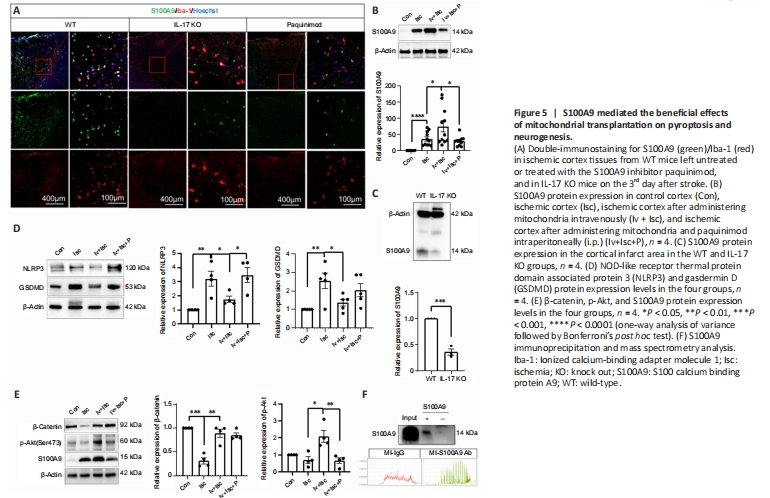
S100A9 protein expression by microglia in the ischemic cortex was increased considerably by ischemic injury, further upregulated by iv mitochondrial transplantation, and decreased by knocking out IL-17 or administering an S100A9 inhibitor (paquinimod) (Figure 5A–C), suggesting that S100A9 is involved in mediating the beneficial effects of mitochondria in this context. NLPR3 and GSDMD protein expression levels in the ischemic cortex were downregulated considerably in response to mitochondrial transplantation, while β-catenin and p-Akt were substantially upregulated, suggesting that mitochondrial transplantation inhibited pyroptosis and promoted neurogenesis. Interestingly, the effect of mitochondrial transplantation on NLRP3 and p-Akt expression was reduced by administration of an S100A9 inhibitor (Figure 5D and E), indicating that S100A9 may mediate the effects of exogenous mitochondria. Co-IP with an anti-S100A9 antibody and subsequent MS analysis identified a number of proteins that interacted specifically with S100A9, including Prx1, drebrin (DBN1), Syngap1, Septin6, Alyref2, Krt86, and Krt33a (Figure 5F).
Figure 6|Internalized exogenous mitochondria fused with endogenous mitochondria, improving microglial viability.
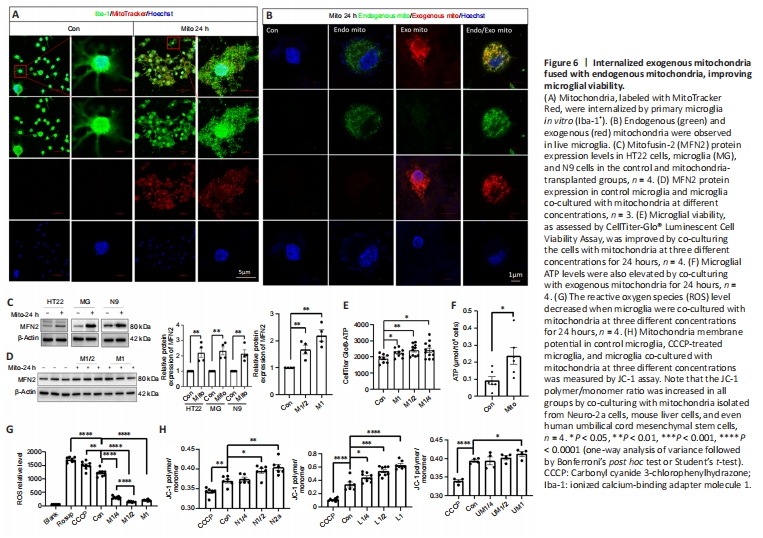
Exogenous mitochondria are easily internalized by neurons, astrocytes, and microglia, and microglia have the greatest ability to do so (Zhao et al., 2021). After in vitro co-incubation for 24 hours, allogeneic mitochondria were easily internalized by Iba-1+ microglia, as indicated by co-localization of endogenous (labeled with MitoTracker Green) and exogenous (labeled with MitoTracker Red) mitochondria in the same microglia (Figure 6A and B), suggesting possible mitochondrial fusion. The endogenous and exogenous mitochondria interacted with each other (Additional Videos). Additionally, an increase in mitochondrial fusion protein MFN2 expression was detected after HT22, microglia, and N9 cells were co-incubated with mitochondria for 24 hours (Figure 6C). MFN2 protein expression also increased in microglia in response to co-incubation with exogenous mitochondria at different concentration for 24 hours (Figure 6D).
CellTiter Glo-ATP and ATP levels in microglia were also increased, and ROS levels were decreased, by administering exogenous mitochondria (M1 group, administration of approximately 5 × 106/mL mitochondria) (Figure 6E–G), indicating the beneficial effects of exogenous mitochondria on redox. Unsurprisingly, mitochondria isolated from Neuro-2a cells, allogeneic cells, and even human umbilical cord mesenchymal stem cells all elevated MMP levels in recipient cells (Figure 6H).
Figure 8|S100A9 promoted microglial internalization of exogenous mitochondria.

Exogenous mitochondria transplantation resulted in downregulation of NLRP3, GSDMD, N-GSDMD, and IL-1β protein expression in microglia subjected to 5 hours of OGD, while β-catenin and Arg-1 were upregulated under the same conditions (Figure 8A–C). S100A9, is a DAMP that is secreted by some myeloid cells in response to certain types of cerebral injury (Srikrishna, 2012), and is commonly phagocytized by microglia (Shichita et al., 2017). S100A9 appears to activate microglia and promote their phagocytic ability (Wu et al., 2018b). In the current study, we found that MFN2 protein expression was upregulated in response to administration of exogenous mitochondria and was further increased by S100A9 overexpression (Figure 8D). GSDMD downregulation and Arg-1 upregulation in response to administration of exogenous mitochondria were significantly suppressed by inhibiting S100A9 (Figure 8E), indicating that S100A9 is crucial to mediating the effects of exogenous mitochondria on microglia. Unsurprisingly, the signal intensity of exogenous mitochondria labeled with MitoTracker Red in N9 cells overexpressing S100A9 and subjected to OGD was significantly higher compared with the control and S100A9 RNAi groups (Figure 8F). Furthermore, significantly more exogenous mitochondria were observed in N9 cells overexpressing S100A9 than in the control and S100A9 RNAi groups (Figure 8G). These results suggest that S100A9 overexpression promotes internalization or endocytosis of exogenous mitochondria by microglia.