NRR:空军军医大学的杨倩研究团队提出线粒体移植治疗局灶性脑缺血有临床转化意义
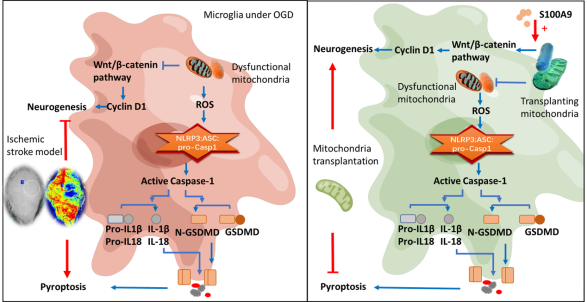
近期,空军军医大学第二附属医院的杨倩团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Mitochondrial transplantation confers protection against the effects of ischemic stroke by repressing microglial pyroptosis and promoting neurogenesis”的研究论文。通过研究,作者系统观察了经静脉移植的线粒体对局灶性脑缺血小鼠死亡率、神经功能、缺血皮质内细胞焦亡和神经发生的影响,并探索了S100A9对线粒体移植功能的调节作用;进而,证明了线粒体移植对体外培养的小胶质细胞的线粒体功能、极化、焦亡和存活通路的影响,以及S100A9对小胶质细胞内化能力的调节。此研究可以进一步补充线粒体移植功效的研究,提出潜在调控靶点,并可能为线粒体移植将来的临床转化提供理论和实验支撑。
缺血性脑卒中是世界范围内的主要致死和终身致残的原因[1, 2],而现有的治疗手段非常有限,故亟需探索新的治疗策略来有效恢复脑功能[1, 3]。线粒体功能异常是脑卒中后神经细胞损伤和死亡的主要特点之一,代谢异常的线粒体,降低的ATP和活性氧自由基(reactive oxygen species,ROS)的异常累积均会引发神经炎症[4, 5]。近年来线粒体移植逐渐成为缺血损伤治疗的热点[2, 6-8],例如,在小鼠短暂局灶性脑缺血模型中,星形胶质细胞内的线粒体可以进入邻近神经元,促进神经元的存活[9]。在大脑中动脉闭塞的大鼠脑卒中模型中,移植到侧脑室内的线粒体可通过抑制细胞氧化应激和凋亡,减少脑梗死面积,来逆转神经功能缺损,甚至促进脑卒中后后期的神经发生[10]。甚至已有两项临床研究证实了自体线粒体移植在心肌缺血再灌注损伤患儿中应用的成功[11, 12]。因此,移植新鲜的、有功能的外源线粒体对于治疗缺血性疾病具有良好的应用前景[2, 7, 8],但采取便于临床转化的静脉给药方式对于局灶性永久性缺血损伤是否具有保护作用,以及其有效的调控方式仍不明确。
研究方法:
1.构建小鼠光血栓局灶性脑缺血模型,观察经尾静脉移植后内化的线粒体对小鼠脑卒中后早期死亡率、焦虑样行为和记忆力的改善;
2.进而通过生物化学、分子生物学和转录组测序等方法系统评价经尾静脉移植的线粒体对小鼠皮质梗死面积、焦亡和神经发生的影响;
3.结合生物信息学分析的方法筛选线粒体移植后表达变化显著的潜在调控分子S100A9,并验证其对线粒体神经保护作用的调节,及可能与其相互作用的蛋白;
4.体外通过多种功能实验和转录组测序方法证明内化的线粒体对小胶质细胞线粒体功能、小胶质细胞极化、焦亡和存活通路的影响;
5.体外验证了S100A9对线粒体移植作用的调控可能源于其对小胶质细胞内化能力的改变。
研究结果证明(1)线粒体移植可显著降低脑卒中后小鼠的死亡率,促进焦虑样情绪和认知功能的恢复,减少脑皮质梗死面积,抑制皮质的焦亡,并促进神经发生;(2)缺血损伤引起小胶质细胞显著增加的S100A9可调节移植线粒体的功效;(3)在培养的小胶质细胞内,外源线粒体在与内源线粒体融合后,能增强线粒体功能、降低氧化还原应激、调节小胶质细胞极化,并抑制焦亡;(4)S100A9可影响外源线粒体在小胶质细胞的内化,从而增强了其促增殖和抗焦亡的作用。总之,线粒体移植通过抑制焦亡和促进神经发生对缺血性卒中发挥保护作用,而且S100A9在促进外源线粒体内化方面起着至关重要的作用。
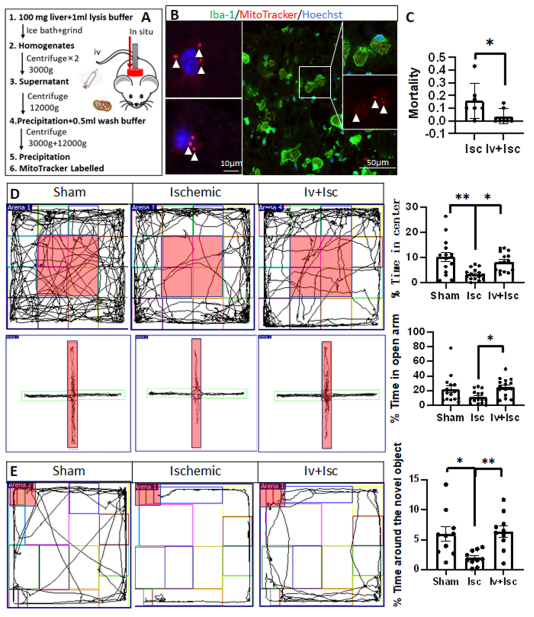
图片说明:线粒体移植改善脑卒中后小鼠的死亡率和异常行为
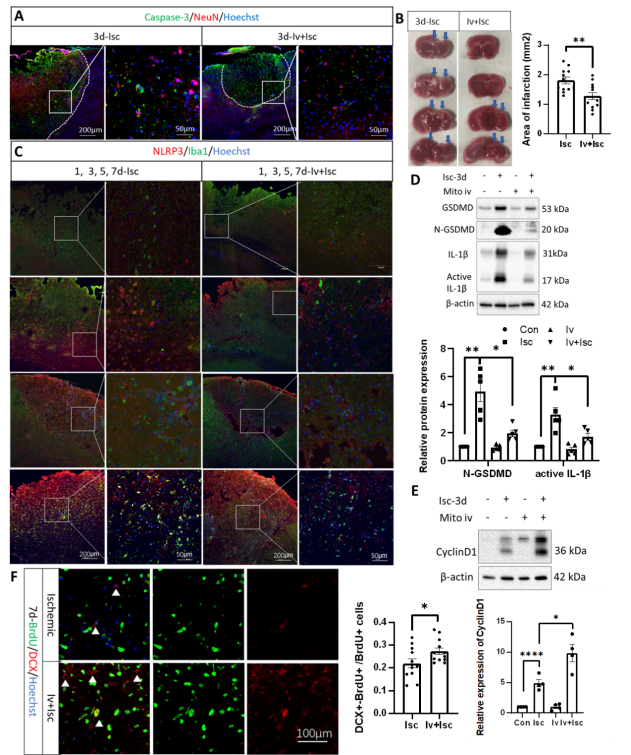
图片说明:线粒体移植可显著缩小缺血皮质的梗死面积、抑制焦亡并促进神经发生
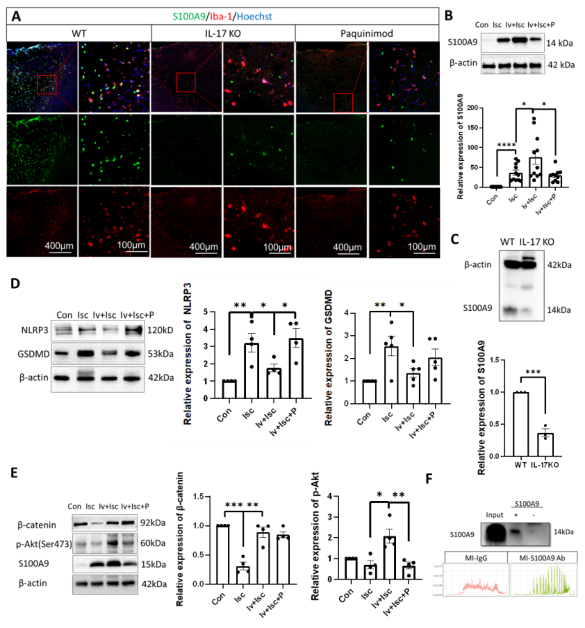
图片说明:S100A9参与调控了线粒体移植的抑焦亡和促神经发生作用

图片说明:内化的线粒体可以小胶质细胞内源性线粒体融合,并提高线粒体功能
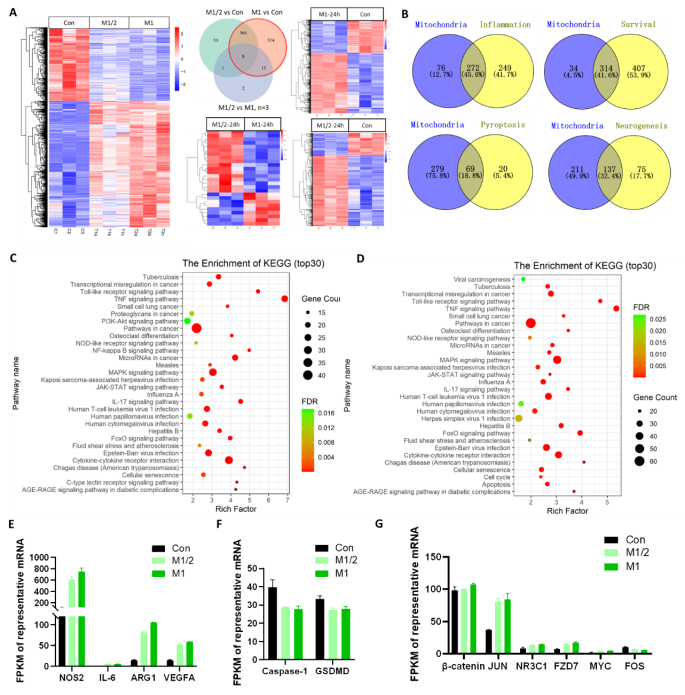
图片说明:移植的线粒体对培养的小胶质细胞系极化、焦亡和生存通路的影响
图示摘要
团队介绍

杨倩,空军军医大学第二附属医院副院长,前沿医学创新中心负责人。当选第十三届全国人大代表,入选国家“万人计划”领军人才、科技部中青年科技创新领军人才。第十二届中国青年女科学家奖、入选军队高层次科技创新人才工程学科拔尖人才、空军级专家,享受军队优秀专业技术人员一类岗位津贴,荣立个人三等功一次,荣获陕西省科学技术进步奖一等奖(第一完成人)、第十届陕西青年科技奖,当选陕西省”三秦学者”岗位特聘专家、陕西省科技创新团队负责人。担任全军医学会神经生物专业委员会常委,中国神经科学学会神经退行性疾病分会常委。主持国家自然科学基金重点项目、重点国际(地区)合作研究项目、面上项目、科技部国家重点研发计划(课题负责人)及省级、军队等课题以及人才建设项目等十余项。主要从事应激条件下神经元稳态的调节及稳态失衡在神经退行性疾病中作用的研究。近年来于Science、Molecular Cell、Nature Communications、 Autophagy等国际知名杂志上发表SCI。

孙丽,空军军医大学第二附属医院在站博士后,获批国家自然科学基金青年项目、陕西省自然科学基金、陕西省中医药中青年科技骨干项目和空军军医大学第二附属医院国科金助推计划等基金。
参考文献:
[1] STINEAR C M, LANG C E, ZEILER S, et al. Advances and challenges in stroke rehabilitation [J]. Lancet Neurol, 2020, 19(4): 348-60.
[2] CHEN W, HUANG J, HU Y, et al. Mitochondrial Transfer as a Therapeutic Strategy Against Ischemic Stroke [J]. Translational stroke research, 2020, 11(6): 1214-28.
[3] CHAMORRO Á, DIRNAGL U, URRA X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation [J]. Lancet Neurol, 2016, 15(8): 869-81.
[4] REUTZEL M, GREWAL R, DILBERGER B, et al. Cerebral Mitochondrial Function and Cognitive Performance during Aging: A Longitudinal Study in NMRI Mice [J]. Oxidative medicine and cellular longevity, 2020, 2020(4060769.
[5] KAUPPILA T E S, KAUPPILA J H K, LARSSON N G. Mammalian Mitochondria and Aging: An Update [J]. Cell Metab, 2017, 25(1): 57-71.
[6] NAKAMURA Y, LO E, HAYAKAWA K. Placental Mitochondria Therapy for Cerebral Ischemia-Reperfusion Injury in Mice [J]. Stroke, 2020, 51(10): 3142-6.
[7] BORLONGAN C, NGUYEN H, LIPPERT T, et al. May the force be with you: Transfer of healthy mitochondria from stem cells to stroke cells [J]. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 2019, 39(2): 367-70.
[8] LU M, GUO J, WU B, et al. Mesenchymal Stem Cell-Mediated Mitochondrial Transfer: a Therapeutic Approach for Ischemic Stroke [J]. Transl Stroke Res, 2021, 12(2): 212-29.
[9] HAYAKAWA K, ESPOSITO E, WANG X, et al. Transfer of mitochondria from astrocytes to neurons after stroke [J]. Nature, 2016, 535(7613): 551-5.
[10] ZHANG Z, MA Z, YAN C, et al. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury
[J]. Behavioural brain research, 2019, 356(322-31.
[11] EMANI S, PIEKARSKI B, HARRILD D, et al. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury [J]. The Journal of thoracic and cardiovascular surgery, 2017, 154(1): 286-9.
[12] GUARIENTO A, PIEKARSKI B L, DOULAMIS I P, et al. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury [J]. J Thorac Cardiovasc Surg, 2021, 162(3): 992-1001.







