视神经损伤
-
Figure 2|Homer1a may be associated with damage to the retina after retinal I/R injury.

To investigate the effect of I/R injury on the retina and Müller cells at different time points, retinal thickness and Müller cell viability were examined after I/R injury. Hematoxylin-eosin staining showed that compared with that in the sham group, retinal thickness began to decrease at 4 hours and had decreased by half at 24 hours after reperfusion (Figure 2A and B). In vitro experiments showed that Müller cell viability was decreased by half compared with that in the control group (Figure 2C) and Müller cell body size shrank and became round (Figure 2D) at 24 hours after I/R injury.
To investigate the changes in Homer1a expression, the transcriptional and translational levels of Homer1a were detected at different time points after reperfusion. Western blotting showed that the expression of Homer1a was increased at 4 hours and peaked at 24 hours after reperfusion in vivo (Figure 2E and F) and in vitro (Figure 2G and H). The changes in Homer1a mRNA expression were consistent with the changes in protein levels, peaking at 24 hours after reperfusion both in vivo (Figure 2I) and in vitro (Figure 2J). Therefore, reperfusion for 24 hours was used in subsequent in vivo and in vitro experiments. These results suggest that changes in Homer1a expression may be associated with retinal damage after I/R injury.
Figure 3|Homer1a protects against damage to retinal tissue and Müller cells after retinal I/R injury.
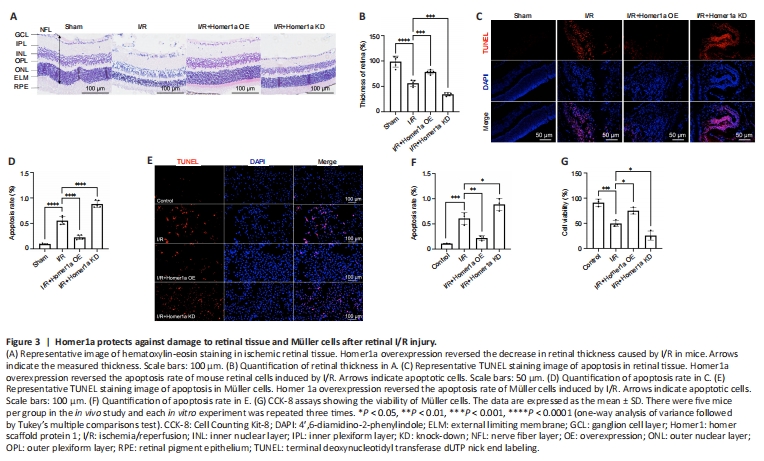
To examine the role of Homer1a, Homer1a OE and Homer1a KD mice were successfully constructed by infecting the mice retina with AAVs, and the efficiency of the virus was verified by RT-PCR, western blotting and fluorescence analysis (Additional Figure 2A–E). In addition, Homer1a OE and Homer1a KD Müller cells were successfully constructed using lentiviral vectors, and the efficiency of the virus was verified (Additional Figure 2F–J). Hematoxylin-eosin staining of retinal paraffin sections showed that retinal thickness was significantly increased in the I/R + Homer1a OE group but was decreased in the I/R + Homer1a KD group compared with that in the I/R group at 24 hours after reperfusion (Figure 3A and B). TUNEL analysis showed that the number of apoptotic retinal cells in Homer1a OE mice after I/R injury was significantly less than that in Homer1a KD mice (Figure 3C and D). In vitro, the TUNEL assay showed that compared with that in the I/R group, the number of apoptotic cells was decreased in the Homer1a OE group and increased in the Homer1a KD group after I/R injury (Figure 3E and F). The CCK-8 results also confirmed that Homer1a OE alleviated the decrease in cell viability after I/R injury, whereas Homer1a KD worsened viability (Figure 3G). These results preliminarily showed that the decrease in retinal tissue thickness and the increase in Müller cell apoptosis after I/R injury were alleviated by Homer1a.
Figure 4|Expression of inflammatory factors after retinal I/R injury is regulated by Homer1a.

The inflammatory response is induced by retinal I/R injury and is one of the main causes of poor disease prognosis (Lin et al., 2019). It has been shown that Homer1a is a promising therapeutic target in the treatment of chronic inflammatory pain (Tappe et al., 2006). To investigate whether Homer1a plays a protective role by regulating inflammatory factors after retinal I/R injury, an unbiased inflammatory gene array containing 200 test indicators was used to determine the expression levels of inflammatory factors in mice retinal tissue. The results showed that basic fibroblast growth factor (bFGF), chemokine (C-X-C motif) ligand 1 (KC/CXCL1), macrophage colony stimulating factor (M-CSF), tumor necrosis factor receptor 1 (TNFR1/TNFRSF1A) and tumor necrosis factor receptor 2 (TNFR11/TNFRSF1B) had the largest changes between the four groups (Figure 4A and B). Compared with those in the I/R group, the expression of these five inflammatory factors was higher in the I/R + Homer1a KD group and lower in the I/R + Homer1a OE group (Figure 4C–G). This finding suggested that Homer1a may play a protective role by decreasing the expression of bFGF, KC/CXCL1, M-CSF, TNFR1/TNFRSF1A, and TNFR11/TNFRSF1B after retinal I/R injury.
Figure 10|Homer1a protein combined with JSH-23 alleviates retinal and Müller cell injury.
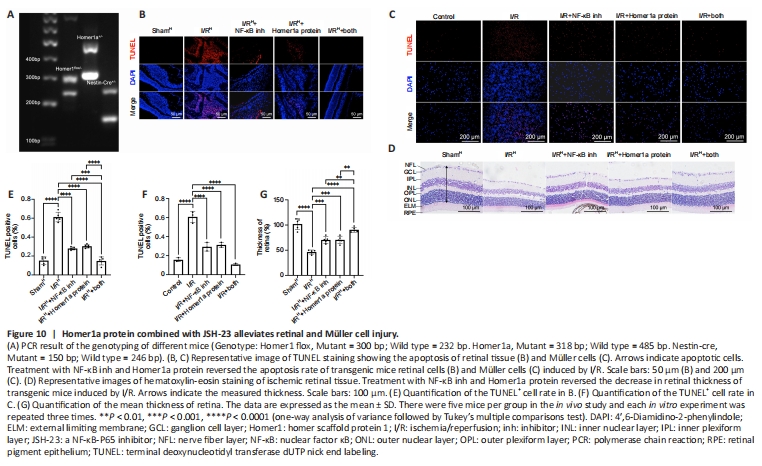
JSH-23 decreases inflammatory mediators and protects the retina during diabetes (Homme et al., 2021). On the basis of our results above, we hypothesized that retinal damage could be alleviated by the combination of Homer1a protein and JSH-23. First, the drug toxicity of the Homer1a protein and JSH-23 was examined by hematoxylin-eosin staining and CCK-8 assay. The use of Homer1a protein and JSH-23 alone or in combination had no adverse effects on retinal tissues (Additional Figure 4A and B) and did not alter Müller cell viability (Additional Figure 4C). To confirm the neuroprotective effect of Homer1a, Homer1flox/?/Homer1a+/?/Nestin-Cre+/? mice were generated and subjected to genetic identification by PCR (Figure 10A). Homer1flox/?/Homer1a+/?/Nestin-Cre+/? mice were intravitreally injected with Homer1a protein and JSH-23. TUNEL assay showed that either Homer1a protein or JSH-23 alone effectively reduced the number of apoptotic cells in retinal tissue after I/R injury, and the combination of the two had a larger effect (Figure 10B and E). Hematoxylin-eosin staining showed that the use of Homer1a protein or JSH-23 alone alleviated the reduction in retinal thickness induced by I/R injury, and the combination of the two had a greater effect (Figure 10D and G). In addition, Homer1a protein plus JSH-23 reduced I/R injury-induced apoptosis in Müller cells (Figure 10C and F). These results showed that Homer1a protein combined with JSH-23 alleviated retinal and Müller cell I/R injury.