NRR:空军军医大学第一附属医院费霏团队发现Homer1a在视网膜缺血再灌注损伤后发挥神经保护作用的机制
撰文:豆雅楠,费晓炜,何鑫,郇宇,魏嘉良,武秀权,吕伟豪,费舟,李侠,费霏
视网膜缺血再灌注损伤是许多视网膜疾病的病理基础[1],如视网膜血管闭塞、糖尿病视网膜病变和眼外伤等。抗氧化剂、钙拮抗剂、抗凋亡剂和神经营养因子在内的多种治疗措施已经用于视网膜缺血再灌注损伤,但是这些疗法的临床疗效确不尽人意[2]。有研究表明,视网膜缺血再灌注损伤的主要原因是炎症级联反应的启动[3-5],其中NACHT-LRR-PYD结构域蛋白3(NLRP3)起着重要作用。NLRP3可通过病原体相关或损伤相关的分子模式直接参与核因子κB(NF-κB)的激活。Homer支架蛋白1(Homer1)是一种神经元突触后支架蛋白,与细胞信号转导和神经元活动密切相关。Homer1蛋白由2大类组成:长型和短型Homer1蛋白。其中短型Homer1蛋白也被称为Homer1a。有报道发现,皮质神经元在氧糖剥夺诱导后,Homer1a表达上调;而过表达Homer1a可减轻氧糖剥夺神经元中乳酸脱氢酶的释放和细胞死亡[6]。然而,对于视网膜缺血再灌注损伤后Homer1a与NLRP3诱导的炎症之间的关系尚知之甚少。此外,视网膜中Müller细胞约占视网膜神经胶质细胞的90%,这些细胞不仅能维持视网膜的正常结构和功能,还能调节视网膜神经元活动并参与各种病理过程。因此,有必要利用Müller细胞构建视网膜缺血再灌注损伤模型,研究Homer1a、NLRP3与炎症的关系,这将为未来视网膜缺血再灌注损伤的治疗提供理论基础。
最近来自中国空军军医大学第一附属医院费霏团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Homer1a reduces inflammatory response after retinal ischemia/reperfusion injury”的研究。该研究发现,缺血再灌注损伤后,Homer1a可通过Caspase-8/NF-κB P65/NLRP3通路对视网膜组织和Müller细胞发挥保护作用。此外,Homer1a联合NF-κB P65抑制剂JSH-23可显著缓解缺血再灌注损伤后视网膜厚度的减少和Müller细胞的凋亡。这对于理解视网膜缺血再灌注损伤后病理变化的机制和确定潜在的治疗策略具有重要意义。
眼内压升高致使NLRP3炎症小体激活,严重情况下可导致视觉受损,是视网膜缺血再灌注损伤的原因之一。有研究报道,Homer1a在大脑神经炎症中发挥保护作用。然而,在眼内压升高引起的视网膜缺血再灌注损伤中,Homer1a对NLRP3炎症小体的影响以及潜在机制尚不清楚。费霏等使用了C57BL/6J和Homer1flox/-/Homer1a+/-Nestin-Cre+/-小鼠构建了由眼内压升高诱导的视网膜缺血再灌注损伤模型,同时在体外以Müller细胞构建了氧糖剥夺再灌注损伤模型。通过病理学研究,小鼠炎症因子芯片、免疫共沉淀等分子生物学检测方法,观察了改变Homer1a表达水平和缺血再灌注损伤后的病理指标、炎症因子水平、NLRP3和Caspase-8炎症小体的激活水平以及NF-κB P65的核转位,还检测了Homer1a蛋白和NF-κB P65抑制剂JSH-23对视网膜组织的厚度和Müller细胞活力的影响。结果发现,Homer1a过表达可抑制缺血再灌注损伤后视网膜厚度的减少和Müller细胞活性的降低。敲低Homer1a可通过Caspase-8促进NF-κB-P65的激活、NF-κB P65的核转位、NLRP3炎症小体的形成以及白细胞介素1β和白细胞介素18的产生和加工,而Homer1a过表达则获得了相反的结果。最后发现,Homer1a和JSH-23联合治疗可显著缓解缺血再灌注损伤后Homer1flox/-/Homer1a+/-Nestin-Cre+/-小鼠视网膜厚度的减少和Müller细胞的凋亡。总而言之,Homer1a在缺血再灌注损伤后通过Caspase-8/NF-κB P65/NLRP3通路对视网膜组织和Müller细胞发挥保护作用(图1)。
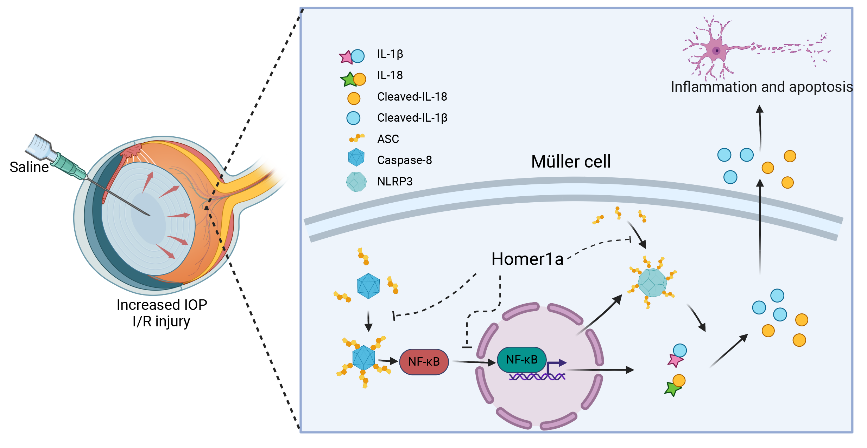
图1 Homer1a可通过抑制Caspase-8/NF-κB P65/NLRP3通路保护Müller细胞免受缺血再灌注损伤(图源:Dou et al., Neural Regen Res, 2024)
上述研究为视网膜缺血再灌注损伤的临床治疗提供了理论依据。当然研究也存在一定的局限性。Müller细胞是分布在视网膜各层的大型神经胶质细胞,在视网膜保护、营养和代谢中发挥重要作用。然而,视网膜分为10层,目前的研究结果尚不清楚Homer1a靶向哪种细胞在体内发挥治疗作用。因此在未来的研究中,可构建具有不同细胞特异性且条件性敲除Homer1a的转基因小鼠,如Homer1flox/-/Homer1a+/-/GFAP-Cre+/-和Homer1flox/-/Homer1a+/-/Cx3Cr1-Cre+/-小鼠,以此来研究Homer1a在视网膜缺血再灌注损伤中的靶向性。
原文链接:https://doi.org/10.4103/1673-5374.386490
参考文献
[1] Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359-393.
[2] Mathew B, Ravindran S, Liu X, et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146-160.
[3] Roche SL, Ruiz-Lopez AM, Moloney JN, et al. Microglial-induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia. 2018;66(2):295-310.
[4] Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14):e93751.
[5] Rivera JC, Dabouz R, Noueihed B, et al. Ischemic retinopathies: oxidative stress and inflammation. Oxid Med Cell Longev. 2017;2017:3940241.
[6] Wei J, Wu X, Luo P, et al. Homer1a attenuates endoplasmic reticulum stress-induced mitochondrial stress after ischemic reperfusion injury by inhibiting the PERK pathway. Front Cell Neurosci. 2019;13:101.
第一作者:豆雅楠,空军军医大学第一附属医院神经外科实验室助理研究员,从事神经元损伤相关领域的科研工作。
并列第一作者:费晓炜,中国人民解放军空军军医大学博士研究生,研究方向为脑外伤相关领域的基础研究。
并列第一作者:何鑫,中国人民解放军空军军医大学硕士研究生,从事神经科学相关领域的基础研究。
通讯作者:费霏,空军军医大学第一附属医院眼科副教授,从事视网膜缺血缺氧领域的相关研究。
并列通讯作者:李侠,空军军医大学第一附属医院神经外科,副教授,副主任医师,从事神经科学领域的相关研究。
并列通讯作者:费舟,空军军医大学第一附属医院神经外科,教授,主任医师,从事神经科学领域的相关研究。
