脑损伤
-
Figure 1|Two-photon imaging of the direct conversion of proliferating glial cells to neurons following retrovirus-mediated NeuroD1 overexpression in the adult mouse cortex.

While many studies have used immunostaining to show that overexpression of neural transcription factors in glial cells can convert them into neurons (Grande et al., 2013; Niu et al., 2013; Guo et al., 2014; Heinrich et al., 2014; Su et al., 2014; Liu et al., 2015; Torper et al., 2015; Gascón et al., 2016; Matsuda et al., 2019; Qian et al., 2020; Lentini et al., 2021; Xiang et al., 2021), the precise conversion process has yet to be observed using a more direct imaging approach. To investigate the in situ glia-to-neuron conversion process, we visualized glial cells overexpressing the transcription factor NeuroD1 (Guo et al., 2014) in the mouse somatosensory cortex for several weeks using two-photon live imaging. All of the two-photon images were acquired through a permanent open-skull cranial window (Figure 1A) (Holtmaat et al., 2009). In order to keep track of the cells of interest during consecutive imaging sessions, blood vessels on the surface of the mouse cortex were used as landmarks to relocate previously imaged areas (Figure 1A) (Holtmaat et al., 2009; Yang et al., 2010). Because retroviruses only express target genes in dividing cells like proliferating glial cells, but not non-dividing cells such as neurons (Katz et al., 2005), we used the retroviral vector CAG::NeuroD1-P2A-GFP to achieve glial cell-specific expression of NeuroD1 in the mouse cortex. The dividing glial cells transfected with the CAG::NeuroD1-P2A-GFP retrovirus in the mouse cortex were actually quite rare (< 1%), consistent with a previous report of only a small population of dividing glial cells in adult mouse brains (Ge and Jia, 2016). Since retroviral expression of NeuroD1 under the chicken beta-actin (CAG) promoter was very strong, we observed a rapid conversion process that occurred over 7–9 days. The control retrovirus CAG::GFP infected dividing glial cells as expected, and virtually all GFP-expressing glial cells retained normal glial morphology throughout the imaging period (Figure 1B). In contrast, many glial cells infected with the CAG::NeuroD1-P2A-GFP retrovirus exhibited rapid morphological changes during the first 3–9 days post injection (dpi) (Figure 1C and D). More specifically, a NeuroD1-overexpressing glial cell would retract some of its glial processes and extend one, or occasionally two, long axon-like neurites to adopt a neuron-like morphology within 7–9 days (Figure 1C and D). Following two-photon live imaging, brain samples were fixed and immunostained to confirm that many of those neuron-like cells were indeed NeuN+ neurons by 10–12 dpi (Figure 1E and F). Moreover, quantification of the retrovirus-infected cells from the two-photon microscopy images revealed that ~40% of the NeuroD1-GFP-expressing cells displayed neuronal morphology with long neurites by 5–7 dpi, and >70% by 7–9 dpi, while only a small percentage retained glial morphology (Figure 1G, n = 10 animals, Ftime (2, 36) = 77.41, P < 0.0001, Fgroup (1, 18) = 139.1, P < 0.0001). Lastly, quantification of the retrovirus-infected cells immunostained with an anti-NeuN antibody after two-photon imaging showed that ~30% of the NeuroD1-GFP-expressing glial cells had converted into NeuN+ neurons by 5–7 dpi, and ~60% by 7–9 dpi (Figure 1H, n = 10 animals, Ftime (2, 36) = 50.25, P < 0.0001, Fgroup (1, 18) = 116, P < 0.0001). These results suggest that at least 60% of the dividing glial cells transfected with the retrovirus CAG::NeuroD1-P2A-GFP eventually converted directly into NeuN+ neurons in adult mouse brains. Thus, the in situ glia-to-neuron conversion process was observed in real time in this study, and our results demonstrate unambiguously that proliferating reactive glial cells can be directly converted into neurons by NeuroD1 overexpression in the adult mouse cortex.
Figure 2|Direct in vitro conversion of astrocytes isolated from astrocytic lineage–tracing mice to neurons following retrovirus-mediated NeuroD1 overexpression.
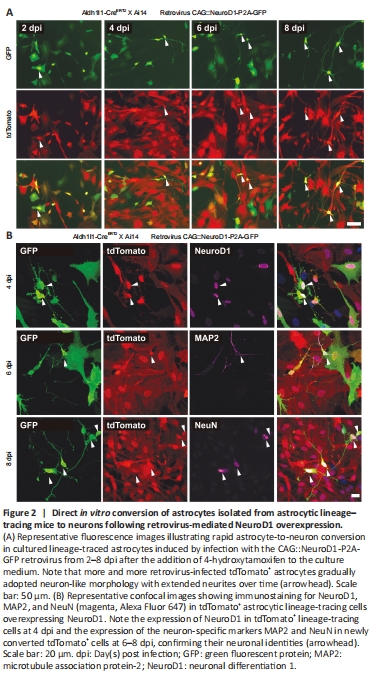
To validate that proliferating astrocytes, a major subtype of proliferating glial cells involved in gliosis (Buffo et al., 2008), can be directly converted into neurons by NeuroD1 overexpression, we examined NeuroD1-induced astrocyte-to-neuron conversion in cultured proliferating astrocytes isolated from astrocytic lineage-tracing mice generated by crossing Aldh1l1-CreERT2 mice (Srinivasan et al., 2016) with Rosa-CAG-LSL-tdTomato (Ai14) mice. In the presence of 4-hydroxytamoxifen in the culture medium, the lineage conversion process took place even more rapidly in vitro than in the mouse brain, with some lineage-traced astrocytes already became tdTomato+ neuron-like cells with extended neurites at 4 dpi (Figure 2A). In fact, many tdTomato+ astrocytes had been converted into NeuN+ and MAP2+ (neuron-specific cytoskeletal protein microtubule-associated protein 2) neurons after retroviral expression of NeuroD1 by 6–8 dpi (Figure 2B), suggesting that many of the lineage-converting reactive glia cells we observed in the mouse cortex are proliferating reactive astrocytes.
Figure 3|Two-photon imaging of the direct conversion of lineage-traced astrocytes into neurons following AAV-mediated NeuroD1 overexpression in adult lineage-tracing mice.

Little astrocyte proliferation occurs in healthy or mildly damaged brains (Sofroniew and Vinters, 2010), so retroviral vectors can only infect a very small portion of astrocytes. In order to monitor the in situ astrocyte-to-neuron conversion process in the vast majority of astrocytes in the mouse cortex, we utilized astrocytic lineage-tracing mice created by crossing Aldh1l1-CreERT2 mice with Ai14 mice. After injecting tamoxifen to induce Cre-mediated recombination, around 90–95% of astrocytes were labeled with tdTomato for lineage-tracing purpose (Additional Figure 1). Then, we employed AAV9 GFAP(CMVe)::NeuroD1 to overexpress NeuroD1 in astrocytes in the mouse cortex under the control of the astrocyte-specific promoter GFAP with a CMV enhancer (CMVe). In the vicinity of the AAV injection sites (~2 mm3), around 75% of the tdTomato+ lineage-traced astrocytes were transfected with AAV9 GFAP::GFP or AAV9 GFAP(CMVe)::NeuroD1, as demonstrated by immunostaining for GFP and NeuroD1 (Additional Figure 2). Two-photon microscopy showed that the tdTomato-traced astrocytes infected with AAV9 GFAP::GFP exhibited few changes in morphology or position over 4 weeks of imaging, consistent with the typical lack of astrocyte migration in the adult mouse brain (Figure 3A; Tsai et al., 2012). AAV-mediated NeuroD1 expression induced a gradual transformation of tdTomato-traced astrocytes from astrocytic morphology to neuronal morphology, with distinct long neurites from 7–27 dpi (Figure 3B). Most NeuroD1-overexpressing tdTomato+ astrocytes initially displayed typical stellate morphology (Figure 3B, 7 dpi), but some quickly lost fine processes and started to sprout neurite-like processes ~2 weeks after infection (Figure 3B, 13–25 dpi). Importantly, these lineage-traced astrocytes never retracted all of their processes, but rather retained several major processes that directly elongated into neurites during conversion (Figure 3B, arrowheads). After two-photon imaging, we performed immunostaining to verify the identity of the tdTomato+ cells in the mouse cortex with or without NeuroD1 infection (Figure 3C and Additional Figure 3). As expected, the tdTomato+ cells in the contralateral hemisphere of the brain without NeuroD1 overexpression were all NeuN- astrocytes (Additional Figure 3A, C and E). In contrast, in the AAV9 GFAP(CMVe)::NeuroD1-injected hemisphere, many tdTomato+ cells had acquired neuronal morphology and stained positive for NeuN by 30 dpi (Figure 3C and Additional Figure 3B, D, F), suggesting that these cells were originally astrocytes that had been converted into neurons. Interestingly, immunostaining also showed AAV9 GFAP1.6::NeuroD1-GFP-infected transitional-stage cells with both neuronal and astrocytic properties that stained positively for both GFAP and NeuN (Additional Figure 4). Moreover, many AAV9 GFAP1.6::NeuroD1-GFP-infected GFP+ cells expressed another neuron-specific cytoskeletal protein, MAP2, in their dendrites and cell bodies at 60 dpi (Additional Figure 5A). Some of these newly converted neurons acquired an upper cortical layer identity (layers II–IV, cortical neuron marker Cux1+), while others acquired a deeper cortical layer identity (layers V–VI, cortical neuron marker Ctip2+) at 60 dpi (Additional Figure 5B). Furthermore, quantification of the lineage-traced astrocytes from the two-photon images revealed that > 6% more tdTomato+ astrocytes in the NeuroD1-expressing hemisphere had adopted neuronal morphology compared with the control hemisphere by 19–21 dpi, and > 10% more by 25–27 dpi (Figure 3D, n = 10 animals, Ftime (3, 54) = 134.3, P < 0.0001, Fgroup (1, 18) = 132.2, P < 0.0001). In addition, quantification of the lineage-traced astrocytes immunostained with a NeuN antibody after two-photon imaging showed that consistently more tdTomato+ astrocytes in the NeuroD1-expressing hemisphere had been converted into NeuN+ neurons compared with the control hemisphere (Figure 3E, n = 10 animals, Ftime (3, 54) = 152.6, P < 0.0001, Fgroup (1, 18) = 78.14, P < 0.0001). A simple calculation indicated that approximately 15% of the lineage-traced astrocytes transfected with AAV9 GFAP(CMVe)::NeuroD1 would eventually be directly converted into NeuN+ neurons in adult astrocytic lineage-tracing mouse brains. Collectively, these results show that lineage-traced astrocytes in the adult mouse cortex can be directly reprogrammed into neurons by 4 weeks of NeuroD1 overexpression, with clear transitional stages (gradually losing glial morphology while adopting neuronal morphology), as observed by continuous two-photon live imaging.
Figure 4|Dynamic growth cones and somal migration during astrocyte-to-neuron conversion, as observed by two-photon live imaging.
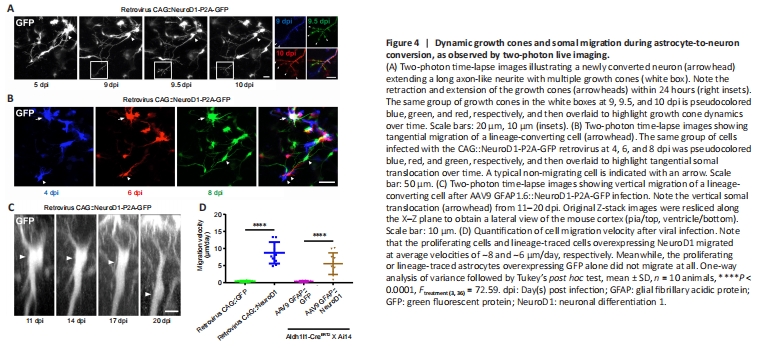
After infection with the retroviral vector CAG::NeuroD1-P2A-GFP, we often observed growth cones at the distal end of newly elongated neurites (Figure 4A, white boxes), reminiscent of newly generated neurons during early brain development. Two-photon images of the newly converted neurons taken every 12 hours suggested that these growth cones were highly dynamic, as they exhibited both extension and retraction (Figure 4A, insets on the right). These observations indicate that the newly converted neurons in the adult mouse cortex extended growth cones to actively explore their local environment. More excitingly, during long-term two-photon imaging, we serendipitously observed the migration of converting cells in the mouse cortex. While endogenous astrocytes in the adult mouse brain rarely migrate due to the formation of gap junctions among adjacent astrocytes, some cells infected with the CAG::NeuroD1-P2A-GFP retrovirus displayed clear migration during time-lapse imaging (Figure 4B). As shown in Figure 4B, the NeuroD1-GFP-expressing cells at different time points were pseudo-colored to track their relative positions, and the images were then overlaid. The arrowheads in the figure indicate a NeuroD1-GFP-expressing cell that exhibited tangential somal translocation between 4 and 8 dpi (Figure 4B). Similarly, when we constructed vertical stacks of two-photon images of NeuroD1-GFP-expressing cells, we found that some of the converting cells also exhibited vertical somal translocation (Figure 4C). Quantification of the migration velocity demonstrated that the converting cells expressing NeuroD1 migrated ~5–8 μm per day, while the non-converting glial cells remained in place during the same time period (Figure 4D, n = 10 animals, Ftreatment (3, 36) = 72.59, P < 0.0001). Together, these data suggest that, like neuronal differentiation and migration during early brain development, the astrocyte-converted neurons can also migrate within the mouse cortex, possibly to find suitable target locations.
Figure 5|Neurite outgrowth and dendritic spine development during astrocyte-to-neuron conversion, as observed by two-photon live imaging.

To determine how fast the neurons induced to convert from glial cells by in situ reprogramming sent out neurites within a pre-existing neural circuit, we examined the time course of neurite outgrowth during astrocyte-to-neuron conversion in the mouse cortex via two-photon live imaging. Figure 5A illustrates a number of traced examples showing the major branches of cells infected with AAV9 GFAP::GFP (red), AAV9 GFAP1.6::NeuroD1-P2A-GFP (blue), or the CAG::NeuroD1-P2A-GFP retrovirus (green) at different time points following viral injection. Astrocytes expressing GFP alone projected many major branches that were relatively short; in contrast, converting cells expressing NeuroD1-GFP showed fewer branches, with one or two very long processes (Figure 5A). To quantify the major branch patterns of the converting cells, we employed Sholl analysis to track the number of major branches at different distances from the center of the soma. Endogenous astrocytes displayed ~10 major branches within a radius of 20 μm. In contrast, AAV9 GFAP1.6::NeuroD1-P2A-GFP-infected cells projected ~6 major branches at 7–9 dpi, which dropped further to < 3 at 19–21 dpi. Along with the retraction of astrocytic processes, the converting cells also sent out a few long branches that kept growing longer after 2–3 weeks of NeuroD1 overexpression (Figure 5B, n = 20 cells, Fgroup (3, 76) = 6.59, P = 0.0006). Similarly, proliferating cells infected with the CAG::NeuroD1-P2A-GFP retrovirus had extended their neurites significantly inside the mouse cortex after ~1 week of NeuroD1 overexpression (Figure 5C, n = 20 cells, Fgroup (2, 57) = 8.16, P = 0.0257). Based on the average neurite length data we collected over a defined period, the average neurite outgrowth speed of the converting cells within a well-established mouse brain circuit was estimated to be around 4 to 40 μm/day.
In addition, many newly converted neurons generated an increasing number of dendritic spines after ~4–8 weeks of NeuroD1 overexpression (Figure 5D and E). The spine density was about 0.6 spine per μm for newly converted neurons originating from proliferating glial cells by 26 dpi, and about 1 spine per μm for newly converted neurons originating from lineage-traced astrocytes by 60 dpi (Figure 5F, n = 10 animals, Ftreatment (3, 36) = 33.86, P < 0.0001).
Figure 6|Functional integration of newly converted neurons into pre-existing neural circuits, as revealed by two-photon Ca2+ imaging in astrocytic lineage-tracing mice.

Using astrocytic lineage-tracing mice (Aldh1l1-CreERT2 X Ai14), we further investigated the functional relationship between astrocyte-converted neurons and pre-existing neurons through two-photon Ca2+ imaging with ultra-sensitive protein calcium sensor green fluorescent protein-calmodulin fusion protein 6 sensitive (GCaMP6s) (Chen et al., 2013). We first injected AAV5 GFAP::GCaMP6s and AAV9 GFAP(CMVe)::NeuroD1 into the neocortex of astrocytic lineage-tracing mice. At 5 dpi, nearly all of the tdTomato+ cells were still astrocytes, and their Ca2+ traces showed typical astrocytic Ca2+ spikes, with slow rising and decaying phases of several seconds’ duration (Figure 6A). Then, we injected AAV5 hSyn::GCaMP6s and AAV9 GFAP(CMVe)::NeuroD1 together into the brain of the lineage-tracing mice. At 30 dpi, we found that some tdTomato+ cells exhibiting spontaneous somatic Ca2+ spikes very similar to those seen in the endogenous neurons (Figure 6B and C). More interestingly, during two-photon time-lapse imaging, we observed that some tdTomato+ astrocyte-converted neurons displayed somatic Ca2+ spikes that were synchronized with those of endogenous neurons (Figure 6D and E), suggesting that these astrocyte-converted neurons had successfully integrated into local neural circuits. Quantification of the rising and decaying time of somatic Ca2+ transients demonstrated much shorter rise-to-peak time and decay time in converted neurons (trise-to-peak ≈ 200 ms, tdecay ≈ 2 seconds) than in resident astrocytes (trise-to-peak ≈ 3.5 seconds, tdecay ≈ 8 seconds, Figure 6F), which is consistent with somatic Ca2+ signals in astrocytes generally occurring at a distinctively slower rate than neuronal Ca2+ events (Wang et al., 2006). In fact, the timescales of somatic Ca2+ transients in converted neurons were indistinguishable from those in endogenous neurons (Figure 6F, n = 10 animals, Ftreatment (5, 54) = 168.4, P < 0.0001), indicating that the newly converted neurons exhibited the same intracellular Ca2+ release features as mature neurons. Taken together, the results from two-photon Ca2+ imaging of astrocytic lineage-tracing mice provide further proof that lineage-traced astrocytes can be converted into functional neurons and then contribute to synchronized neural circuit activity.
Figure 7|Functional synaptic connections formed by the astrocyte-converted neurons, as revealed by electrophysiological recordings.
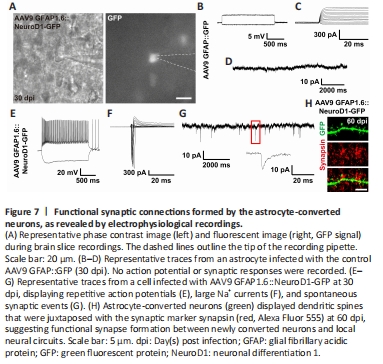
As an alternative approach to investigate the functionality of astrocyte-converted neurons, we tested the electrophysiological properties of the newly converted neurons in cortical slices at 30 dpi using patch-clamp recording (Figure 7A). As expected, AAV9 GFAP::GFP-infected cells did not exhibit any action potentials (Figure 7B), voltage-gated sodium currents (Figure 7C), or spontaneous synaptic responses (Figure 7D), consistent with the properties of typical astrocytes (n = 11). In contrast, many AAV9 GFAP1.6::NeuroD1-GFP-infected cells exhibited repetitive action potentials (Figure 7E), large voltage-gated sodium currents (Figure 7F), and spontaneous synaptic responses (Figure 7G), consistent with the properties of typical neurons (n = 22). Moreover, immunostaining with the synaptic marker synapsin1 at 60 dpi showed presynaptic puncta juxtaposed with the dendritic shafts and dendritic spines of NeuroD1-GFP+ cells (Figure 7H), confirming that the astrocyte-converted neurons did become functionally incorporated into an established neural network in the mouse cortex. Collectively, our two-photon Ca2+ imaging and patch-clamp recording results demonstrate that astrocytes can be directly reprogrammed into fully functional neurons that can integrate into pre-existing neural circuits, and unambiguously show that adult mammalian brains are highly plastic in terms of neuroregeneration and neural circuit remodeling.