脑损伤
-
Figure 1|Neuronal ER stress is significantly induced after TBI in vitro, and inhibition of activated neuronal ER stress protectes against trauma-induced injury.

To investigate the role of ER stress in TBI pathology, we performed scratch damage tests using cultured HT22 neurons. MAP-2 immunofluorescence staining confirmed pure HT22 neuron cultures that were then used as in vitro TBI models (Additional Figure 3A–C). Western blotting showed that GRP78, p-IRE1α, XBP1s, CHOP, cleaved caspase 12, and cleaved caspase 3 expression levels were higher in neurons following scratch damage than in the Control group (Figure 1A–C). Immunofluorescence staining indicated that GRP78, a well-established ER stress biomarker (Ibrahim et al., 2019), was highly expressed in the scratch injury group (Figure 1D and E). These findings indicate that ER stress is related to TBI and leads to neuronal death.
To determine whether suppressing ER stress can alleviate neuronal injury, we treated injured neurons with TUDCA, a commonly used ER stress inhibitor. Then, the neuronal expression levels of GRP78, p-IRE1α, XBP1s, CHOP, cleaved caspase 12, and cleaved caspase 3 following damage and TUDCA treatment were quantified via western blot analysis. In addition, GRP78 levels were examined via immunofluorescence staining. The results showed that all of the expression levels of all tested proteins were suppressed in the group treated with TUDCA compared with the Injury group (Figure 1A–C). The IF results were consistent with the western blot results (Figure 1D and E). Taken together, these findings clearly indicate that scratch injury significantly induces ER stress in neurons in vitro. In addition, the induction of ER stress following TBI may lead to neuronal injury, and inhibiting ER stress may reduce apoptosis and have a neuroprotective function.
Figure 2|Real-time polymerase chain reaction validation of microglial exosomes isolated from mouse brains at different time points after rmTBI.

To investigate the role of microglial exosomal miRNAs in pathological alterations following rmTBI, we employed the rmTBI mouse model (Figure 2A) that we developed previously (Ge et al., 2018). Meanwhile microglial exosomes from the combined tissue of the hippocampus and cerebral cortex following rmTBI were collected by ultracentrifugation for miRNA microarray analysis, which showed clear alterations in miR-124-3p content (Additional Figure 4; Ge et al., 2020). In addition, we used real-time polymerase chain reaction to validate miR-124-3p expression changes in microglial exosomes collected from mouse brains after rmTBI. Nanoparticle tracking analysis showed that the peak diameter of the exosomes was 138 ± 4.0 nm (Figure 2B). The identity of the microglial exosomes was verified by staining with exosome biomarkers (CD9, CD63, CD81, and TSG101) (Figure 2C), which showed that the markers were expressed at high levels in the precipitate but low levels in the supernatant. Exosome morphology was assessed by transmission electron microscopy, which showed that they were round particles with double membranes, with a size range of 50–150 nm (Figure 2D). These results indicate that exosomes were the main component of the microglial precipitants. Real-time polymerase chain reaction demonstrated that all rmTBI groups had elevated miR-124-3p levels in their microglial exosomes (Figure 2E). Elevated miR-124-3p content in microglial exosomes has been demonstrated to protect neurons from damage following TBI. Consequently, miR-124-3p was selected as the key miRNA for further investigation.
Figure 4|Microglia-derived exosomal miR-124-3p inhibits neuronal ER stress by increasing miR-124-3p expression in injured neurons.
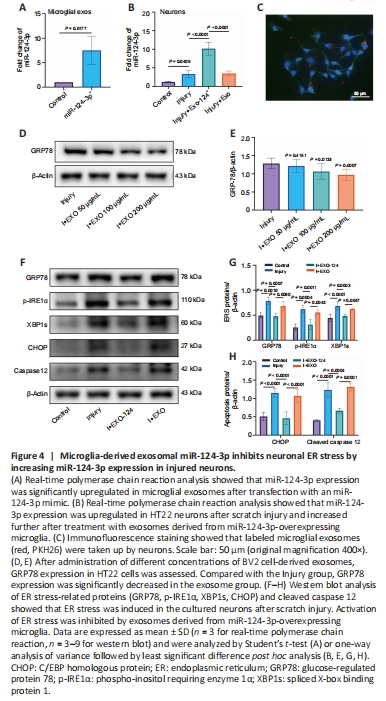
Exosomes derived from microglia overexpressing miR-124-3p or normal microglia were added to HT22 cells to validate the impact of exosomal miR-124-3p on scratch-injured neurons. Additionally, miR-124-3p levels in HT22 cells and microglial exosomes were detected by real-time polymerase chain reaction (Figure 4A and B). The statistical analysis showed that the miR-124-3p expression level rose after scratch damage and was even higher after exposure to exosomes derived from microglia overexpressing miR-124-3p. However, miR-124-3p expression was not altered significantly in damaged neurons exposed to normal exosomes. Next, exosomes derived from microglia overexpressing miR-124-3p were labeled with PKH26 and introduced to the HT22 neuron culture medium; immunofluorescence staining showed that the HT22 cells phagocytosed the exosomes (Figure 4C). To determine the best concentration of microglial exosomes for treating injured neurons, we tested different concentrations (50–200 μg/mL) and performed western blotting to detect GRP78 concentrations. At a concentration of 200 μg/mL,
GRP78 expression in injured neurons decreased significantly, so this concentration was used for the subsequent experiments (Figure 4D and E). Next, we examined the expression of proteins associated with neuronal cell apoptosis after damage and exosome treatment and found that treatment with exosomes derived from miR-124-3p-overexpressing microglia inhibited CHOP and cleaved caspase 12 expression in neurons. Subsequently, we found that the expression levels of the ER stress marker proteins GRP78, p-IRE1α, and XBP1s were also reduced in neurons that exposed to exosomes derived from miR-124-3p-overexpressing microglia compared with those in the Injury group. However, compared with the Injury + EXO-124 group, the expression levels of GRP78, p-IRE1α, XBP1s, CHOP, and cleaved caspase 12 were higher in the Injury + EXO group. This further suggests that the inhibition of ER stress by microglial exosomes is achieved through the exosome-mediated transport of miR-124-3p into neurons (Figure 4F–H). These results suggest that microglia-derived exosomal miR-124-3p suppressed neuronal ER stress in damaged neurons, thereby mitigating neuronal damage.
Figure 6|miR-124-3p reduces apoptosis and promotes proliferation of injured neurons.
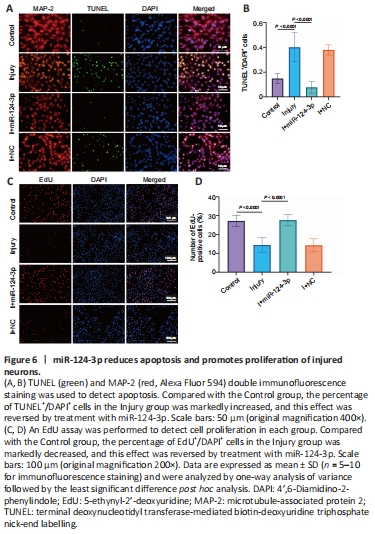
To more clearly validate the impact of miR-124-3p on IRE1α expression in damaged neurons, injured neurons were transfected with an miR-124-3p mimic, a negative control, or an miR-124-3p inhibitor. After 1 day of transfection, real-time polymerase chain reaction analysis showed that the level of miR-124-3p expression was significantly altered in the various transfected groups (Figure 5C and D). In HT22 cells transfected with the miR-124-3p mimic, miR-124-3p expression was clearly greater than in the NC group, but it was lower in HT22 cells transfected with the miR-124-3p inhibitor. In addition, IRE1α expression was significantly lowered by miR-124-3p overexpression, but significantly increased by miR-124-3p inhibition (Figure 5E and F). Next, to evaluate the direct impact of miR-124-3p on ER stress in damaged neurons, we investigated the expression of ER stress-related proteins (p-IRE1α, GRP78, and XBP1s), as well as the expression of CHOP, cleaved caspase 12, and cleaved caspase 3 in each experimental group. Our findings demonstrated that transfection with an miR-124-3p mimic suppressed ER stress and cell death in damaged neurons, while transfection with an miR-124-3p inhibitor somewhat enhanced both ER stress and cell death (Figure 5G–I). TUNEL staining and EdU detection revealed that the apoptosis rate of the damaged cells increased and their proliferation rate diminished compared with the Control group. However, when the damaged cells overexpressed miR-124-3p, the apoptotic rate was reduced, while the rate of cell proliferation was significantly elevated (Figure 6A–D). These findings are consistent with our earlier results. In conclusion, miR-124-3p may help protect neuronal function by directly targeting IRE1α and inhibiting activation of ER stress in injured neurons.
Figure 8| Intranasal delivery of miR-124-3p-enriched microglial exosomes inhibites ER stress and reduced apoptosis in C57BL/6 mice after rmTBI.
To elucidate the suppressive effect of exosomes derived from microglia overexpressing miR-124-3p on ER stress after rmTBI, we administered exosomes containing miR-124-3p to the injured brains of rmTBI mice via intranasal delivery. At 14 days post-rmTBI we quantified GRP78, p-IRE1α, and XBP1s expression by western blot and discovered that the expression of all three proteins was decreased by administration of the exosomes. Similarly, compared with rmTBI group, CHOP and cleaved caspase 12 expression levels were significantly decreased in the rmTBI + EXO-124 group (Figure 8A–C). In addition, double immunofluorescence staining for GRP78 and NeuN clearly demonstrated that there were significantly more GRP78-positive cells in the hippocampus of rmTBI mice than in Sham mice, and that administration of EXO-124 mitigated this effect (Figure 8D and E). This result indicates that microglia-derived exosomes rich in miR-124-3p reduced rmTBI-induced ER stress activation and preserved cellular function. Therefore, EXO-124 treatment alleviated neuronal injury after rmTBI.