脊髓损伤
-
Figure 4|SCI-induced lipid peroxidation and iron deposition aggravate ferroptosis.
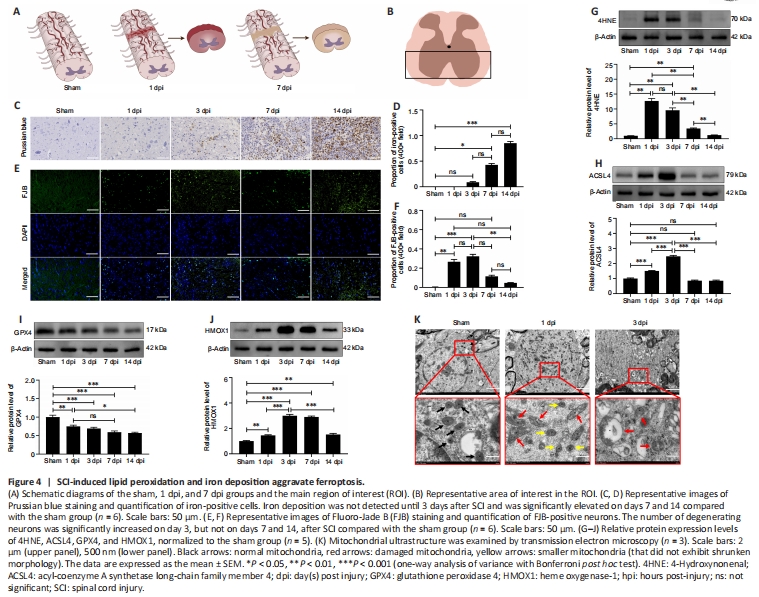
The schematic image shown in Figure 4A depicts the morphological changes observed in the spinal cord on days 1 and 7 after SCI. The representative images were selected from the approximate area of the anterior horn of the spinal cord within the region of interest (Figure 4B). We detected iron deposition starting on day 1 after SCI, and iron deposition levels were significantly elevated a week later (Figure 4C and D). Unlike iron deposition, which appeared later, neuron degeneration was readily apparent within the first 3 days after SCI and did not increase significantly thereafter (Figure 4E and F). Western blotting showed that 4HNE and ACSL4 were upregulated in the first 3 days and returned to near-normal levels on day 7 (Figure 4G and H). GPX4 expression decreased slowly over the follow-up period, while HMOX1 remained upregulated (Figure 4I and J). During the initial 3-day period, we did not observe any mitochondria with typical characteristics associated with ferroptotic cell death; instead, we identified damaged mitochondria showing reduced or absent cristae (Figure 4K). Owing to the inflammatory response, lipid peroxidation and neuronal cell death were more pronounced in the first 3 days after SCI than at later time points, suggesting that this is the appropriate time frame for assessing the role of ferroptosis in SCI.
Figure 5|Met treatment increases HMOX1 expression after SCI.

Figure 6|Met attenuates neuronal cell ferroptosis and neuroinflammation after SCI.
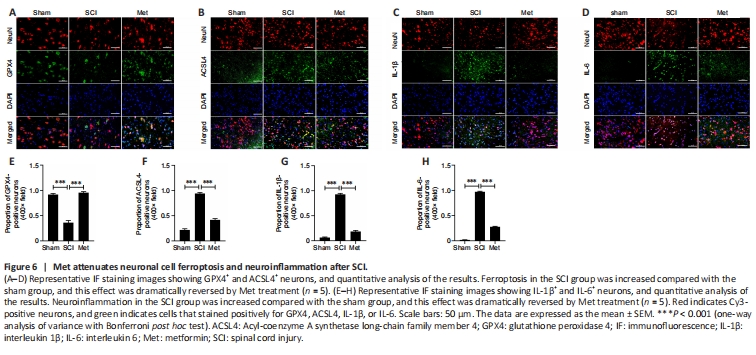
Based on the results of bioinformatics and western blotting analyses, we assessed HMOX1 expression in the gray matter and white matter of the spinal cord at different time points after SCI. The immunohistochemistry results showed that HMOX1 was uniformly and consistently upregulated in the SCI group compared with the sham group (Figure 5A–D). Studies have suggested that GPX4 downregulation and ACSL4 upregulation induce and promote the progression of ferroptosis (Chen et al., 2021b; Liu et al., 2022a). Here, we found that SCI induced a significant decrease in Gpx4 expression on day 3, rather than on day 1, after SCI. In addition, Gpx4 expression was significantly upregulated within the first 3 days after Met treatment (Figure 5E). ACSL4 and HMOX1 were upregulated after SCI, while Met treatment downregulated ACSL4 and upregulated HMOX1 (Figure 5F–I). Met treatment also downregulated the trauma-induced 4HNE expression, further demonstrating that Met effectively regulates lipid peroxidation levels after SCI (Figure 5J). To determine the extent of ferroptotic neuronal cell death at the tissue level, we performed double immunofluorescence labeling of GPX4, ACSL4, IL-1β, and IL-6 with neuronal marker and found that, compared with the SCI group, neurons from rats in the Met treatment group exhibited upregulated GPX4 expression and downregulated ACSL4, IL-1β, and IL-6 expression (Figure 6A–H). This indicated that Met treatment reduced both ferroptosis and inflammation in neurons after SCI. In conclusion, these findings show that Met inhibit ferroptosis at the tissue and neuronal levels.
Figure 8|Met promotes the polarization of M1-type microglia/macrophages towards an M2 phenotype in an HMOX1-independent manner.
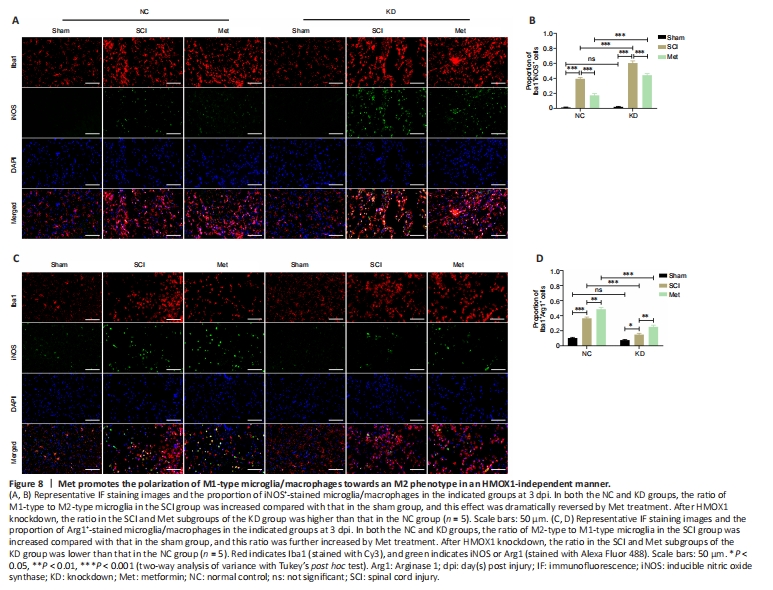
After TSCI, activated M1 microglia release nitric oxide (NO), causing oxidative stress and a neurotoxic cascade in neurons. It is thought that Arg1 blocks uncoupling of nitric oxide synthase (NOS) in M2 microglia (Ma et al., 2017). HMOX1 expression in macrophages is induced by stress and promotes their differentiation into an M2 phenotype, with associated anti-inflammatory activities (Campbell et al., 2021). In both the NC and KD groups, the proportion of Iba1+/iNOS+ microglia/macrophages was significantly upregulated after SCI, and significantly downregulated after Met therapy. In the SCI and Met subgroups (SCI subgroup of the KD group vs. SCI subgroup of the NC group, Met subgroup of the KD group vs. Met subgroup of the NC group), the proportion of Iba1+/iNOS+ microglia/macrophages was significantly upregulated after HMOX1 knockdown. This indicates that HMOX1 upregulation and Met treatment prevented overactivation of M1-type microglia in SCI rats (Figure 8A and B). Similarly, in both the NC and KD groups, the ratio of Iba1+/Arg1+ microglia/macrophages was significantly increased after SCI, and Met treatment further increased this ratio. However, in the SCI and Met subgroups, the ratio of Iba1+/Arg1+ microglia/macrophages was significantly decreased after HMOX1 knockdown. This indicates that HMOX1 upregulation and Met treatment promoted activation of M2-type microglia cells in SCI rats (Figure 8C and D). These findings suggest that both HMOX1 upregulation and Met treatment after SCI can promote the polarization of M1 microglia/macrophages towards an M2 phenotype, and that the effects of Met may not be dependent on HMOX1 upregulation.
Figure 9|Met exerts a neuroprotective effect in an HMOX1-dependent manner.
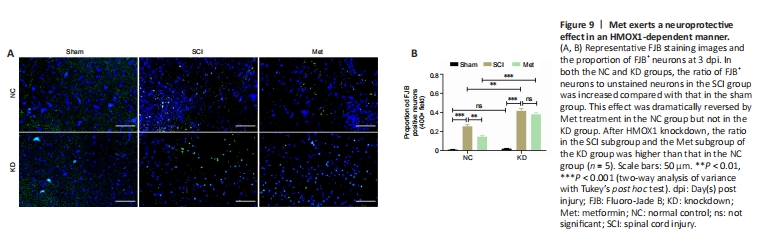
Finally, we examined the effect of HMOX1 knockdown on neuronal damage. As depicted in Figure 9, the proportion of FJB-positive neurons increased significantly following SCI in the NC and KD groups. In the NC group, Met treatment noticeably reduced neuronal damage, but this effect was not pronounced in the KD group. In the SCI and Met subgroup, HMOX1 knockdown led to a considerable increase in neuronal damage. These findings indicate that HMOX1 upregulation following SCI helps attenuate nerve damage, and that the effectiveness of Met treatment partly relies on HMOX1 upregulation.