神经退行性病
-
Figure 2|Rotenone administration induces intestinal barrier disruption in a gut microbiota-dependent manner.

Given that rotenone appeared to alter the gut microbiota, we next asked whether rotenone also perturbed intestinal homeostasis, specifically intestinal barrier integrity, permeability, and inflammation. Our findings showed that rotenone administration resulted in a significant reduction in colonic mucus thickness compared with the Con group (P < 0.001; Figure 2A), as demonstrated by Alcian blue staining (Figure 2A and B) and MUC2 immunofluorescence staining (Figure 2C) of colon tissue. Additionally, rotenone administration significantly decreased the expression levels of tight junction proteins, namely ZO-1 and occludin, in the colon (both P < 0.05, Figure 2D). Consequently, rotenone administration resulted in elevated serum LPS levels, suggesting that rotenone disrupted the integrity of the gut barrier and increased gut permeability to endotoxins (Figure 2E). Furthermore, we observed higher IL-6, TNF-α, and inducible nitric oxide synthase (iNOS) mRNA levels in the colon tissue of mice in the Rot group compared with the Con group (P < 0.05; Figure 2F–H), indicating increased intestinal inflammation. Consistently, we found that a shorter colon length was associated with intestinal inflammation in response to rotenone administration (Figure 2I).
To investigate the role of the gut microbiota in rotenone-induced intestinal barrier disruption and intestinal inflammation, we used a cocktail of oral antibiotics to deplete the effects of the gut microbiota. Interestingly, we found no significant difference in colonic mucus thickness or inMUC2 immunofluorescence staining in the colon between the Con + Ab group and the Rot + Ab group (P > 0.05; Figure 2J–L). Moreover, there were no significant differences between the Con + Ab group and the Rot + Ab group in terms of serum LPS levels, the expression levels of tight junction proteins (ZO-1 and occludin), mRNA expression levels of inflammatory factor (IL-6, TNF-α, and iNOS), and colon length (P > 0.05, Figure 2M–R). Taken together, these findings suggest that the disruption of intestinal barrier integrity and intestinal inflammation that we observed following chronic rotenone administration were dependent on the presence of the gut microbiota.
Figure 3|Mice treated with rotenone exhibit C/EBP/AEP pathway activation, α-syn aggregation, and TH-positive neuron loss in the SN, and these effects are dependent on the gut microbiota.
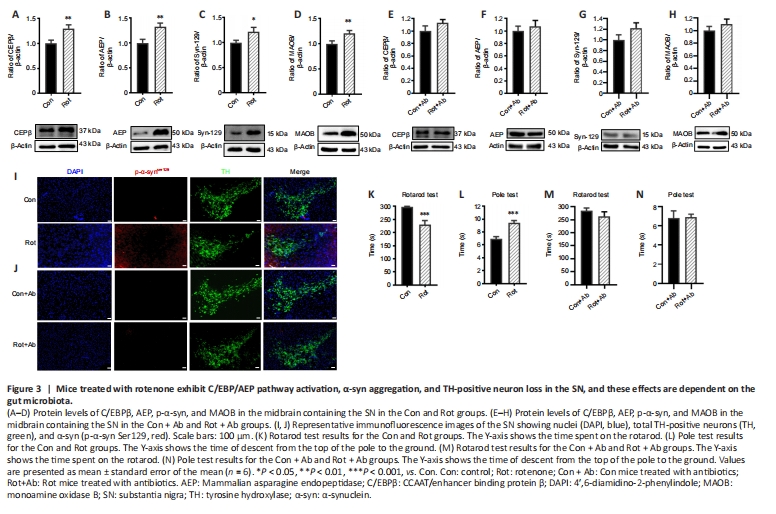
It has been reported that C/EBPβ is activated by LPS (Ejarque-Ortiz et al., 2007) and pro-inflammatory cytokines (Cox et al., 2013; Pulido-Salgado et al., 2015). Given our finding that LPS and inflammatory cytokine levels increased after rotenone administration, we assessed the expression levels of C/EBPβ/AEP signaling molecules in the SN of mice treated with rotenone by western blot. Both C/EBPβ and AEP levels were upregulated in the SN of the Rot group compared with the Con group (P < 0.05; Figure 3A and B). This upregulation in C/EBPβ/AEP signaling was gut microbiota-dependent, since the levels of C/EBPβ and AEP did not differ between the Rot + Ab and Con + Ab groups (Figure 3E and F). Previously, it was reported that the C/EBPβ/AEP signaling pathway regulates transcription and proteolytic cleavage of α-syn and MAOB (Wu et al., 2021). In this study, we found that both α-syn and MAOB protein levels were significantly increased in the Rot group compared with the Con group (P < 0.05, Figure 3C and D), but not in the Rot + Ab and Con + Ab groups (Figure 3G and H). Immunofluorescence staining revealed abnormal α-syn aggregation around tyrosine hydroxylase-positive neurons along with decreased α-syn expression in the SN in the Rot group (Figure 3I), but not in the Rot + Ab group (Figure 3J). As a consequence, mice in the Rot group exhibited motor impairment, a main symptom of PD, as assessed by the rotarod test and pole test (Figure 3K and L). However, this rotenone-induced motor impairment was eliminated by using antibiotics to ablate the gut microbiota (Figure 3M and N). Taken together, these findings suggest that the gut microbiota plays an important role in triggering the CEBP/β-AEP signaling pathway to regulate transcription and proteolytic cleavage of α-syn and MAOB, thereby leading to PD pathogenesis. Spearman’s correlation analysis was used to investigate the relationship between gut bacterial abundance and colonic mucosa thickness, serum LPS, the C/EBPβ/AEP signaling pathway, and motor behavior (Figure 4). We found that Firmicutes abundance positively correlated with motor deficits (r = 0.6979, P < 0.05) and negatively correlated with colonic mucosa thickness (r = –0.8191, P < 0.01) and ZO-1 (r = –5975, P < 0.05). Actinobacteria abundance positively correlated with colonic mucosa thickness (r = 0.7951, P < 0.01). Proteobacteria abundance negatively correlated with motor deficits (r = –0.6594, P < 0.05). Cyanobacteria abundance negatively correlated with motor deficits (r = –0.639, P < 0.05) and ZO-1 (r = –0.6038, P < 0.05) and positively correlated with serum LPS levels (r = 0.6966, P < 0.05). Lachnospiraceae abundance positively correlated with AEP expression level (r = 0.6622, P < 0.05) and negatively correlated with colonic mucosa thickness (r = –0.7957, P < 0.01). Ruminococcaceae abundance positively correlated with motor deficits (r = 0.6976, P < 0.05) and serum LPS levels (r = 0.7899, P < 0.01) and negatively correlated with colonic mucosa thickness (r = –0.7227, P < 0.05). Saccharimonadaceae abundance positively correlated with AEP expression level (r = 0.7972, P < 0.05) and negatively correlated with colonic length (r = –0.9595, P < 0.01). Erysipelotrichaceae abundance negatively correlated with motor deficits (r = –0.6458, P < 0.05) and α-syn expression levels (r = –0.7930, P < 0.01) and positively correlated with colonic mucosa thickness (r=0.8073, P < 0.01). Bifidobacteriaceae and Bifidobacterium abundance positively correlated with colonic mucosa thickness (r = 0.7791, P < 0.01). Desulfovibrionaceae (r = –0.7970, P < 0.01) and Desulfovibrio (r = –0.6683, P < 0.05) abundance negatively correlated with motor deficits. Turicibacter abundance positively correlated with colonic mucosa thickness (r = 0.6046, P < 0.05). Faecalibacterium abundance negatively correlated with motor deficits (r = –0.6214, P < 0.05) and MAOB expression levels (r = –0.7188, P < 0.05) and positively correlated with colonic mucosa thickness (r = 7365, P < 0.01). Dubosiella abundance negatively correlated with α-syn expression levels (r = –0.7182, P < 0.05) and positively correlated with colonic mucosa thickness (r = 0.6047, P < 0.05). These findings suggest that rotenone-induced motor impairment and other elements of PD pathology are associated with disruption of the gut microbiota.
Figure 6| Transplantation of healthy gut microbiota alleviates rotenone-induced motor deficits, intestinal inflammation, endotoxemia, and intestinal barrier impairment.
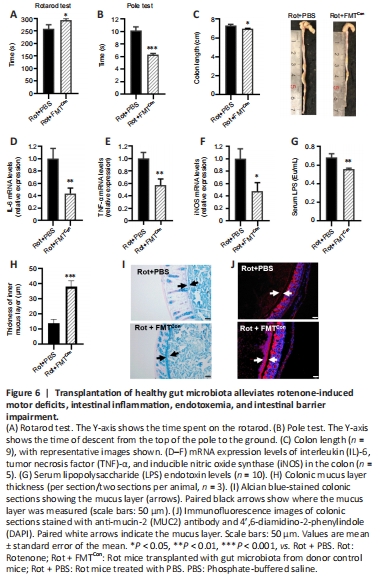
Next, we investigated whether transplanting fecal microbiota from donor Con mice could rescue the motor deficits and intestinal barrier disruption observed in the Rot mice. The motor deficits observed in the rotarod test and pole test were alleviated in Rot mice transplanted with microbiota from donor Con mice compared with those that received PBS (P < 0.05; Figure 6A and B). The rotenone-induced colon shortening (Figure 6C) and increase in colonic IL-6, TNF-α, and iNOS mRNA expression levels (Figure 6D–F) were rescued by gut microbiota transplantation from donor Con mice. Serum LPS levels were decreased in the Rot + FMTCon mice compared with the Rot + PBS mice (P < 0.05; Figure 6G), indicating an improvement in endotoxemia and intestinal mucosal barrier function. Moreover, we observed an increase in colonic mucus thickness, as demonstrated by Alcian blue staining (P < 0.05; Figure 6H and I) and MUC2 immunofluorescence staining (Figure 6J), in the Rot + FMTCon mice compared with the Rot + PBS mice. Therefore, a healthy gut microbiota can alleviate rotenone-induced motor deficits, intestinal inflammation, endotoxemia, and intestinal barrier disruption.