中国神经再生研究(英文版) ›› 2024, Vol. 19 ›› Issue (9): 2081-2088.doi: 10.4103/1673-5374.391191
• 原著:退行性病与再生 • 上一篇
补充健康微生物群可通过激活C/EBPβ/AEP 信号通路改善帕金森病的运动障碍
Gut microbiota dysbiosis contributes to α-synuclein-related pathology associated with C/EBPβ/AEP signaling activation in a mouse model of Parkinson’s disease
Xiaoli Fang1, 2, #, Sha Liu2, #, Bilal Muhammad2, Mingxuan Zheng3, Xing Ge3, Yan Xu2, Shu Kan2, Yang Zhang4, Yinghua Yu3, #br# Kuiyang Zheng3, Deqin Geng2, *, Chun-Feng Liu1, 5, *
- 1Department of Neurology and Clinical Research Center of Neurological Disease, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China; 2Department of Neurology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu Province, China; 3Jiangsu Key Laboratory of Immunity and Metabolism, Department of Pathogen Biology and Immunology, Xuzhou Medical University, Xuzhou, Jiangsu Province, China; 4Department of Neurology, Xuzhou Central Hospital, Xuzhou, Jiangsu Province, China; 5Jiangsu Key Laboratory of Neuropsychiatric Disease and Institute of Neuroscience, Soochow University, Suzhou, Jiangsu Province, China
摘要:
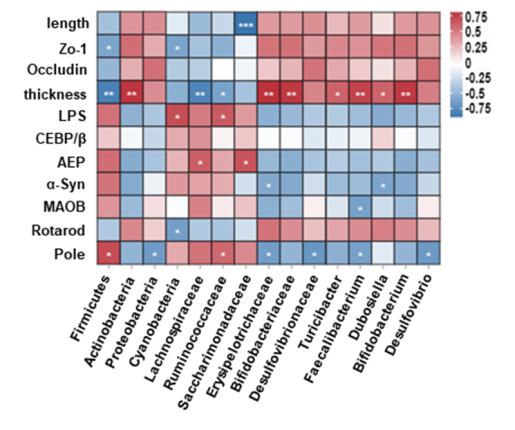
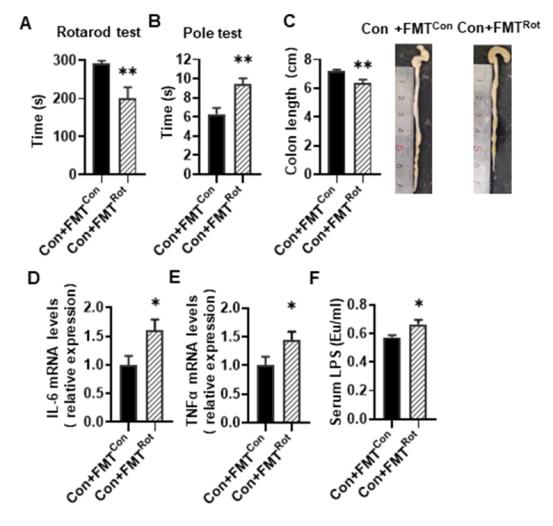
帕金森病胃肠道功能障碍可比运动症状的出现早数年。肠道微生物群失调与帕金森病的发病机制有关,但其在运动功能障碍中的因果作用和机制仍完全未知。细菌内毒素激活的CCAAT/增强子结合蛋白β/天冬酰胺内肽酶(C/EBPβ/AEP)信号转导可促进α-突触核蛋白的转录,从而导致帕金森病的发病。实验旨在利用鱼藤酮诱导的帕金森病小鼠模型,结合抗生素诱导的微生物群耗竭和粪便微生物群移植,研究肠道微生物群在C/EBPβ/AEP信号传导、α-突触核蛋白病理学和运动症状中的作用。实验发现,鱼藤酮会导致小鼠肠道微生物群失调、肠道屏障紊乱、C/EBPβ/AEP通路激活、α-突触核蛋白聚集以及黑质中酪氨酸羟化酶神经元缺失,从而导致小鼠运动障碍。然而,在抗生素预处理的肠道微生物群缺失小鼠中,鱼藤酮没有这些不良影响。重要的是,移植健康小鼠的粪便微生物群可缓解鱼藤酮诱导的运动障碍、肠道炎症、内毒素血症和肠屏障损伤。这些结果表明,肠道微生物群失调在鱼藤酮诱导的帕金森病小鼠模型的运动障碍、C/EBPβ/AEP 信号激活和α-突触核蛋白病理学中的重要作用。说明补充健康的微生物群可为改善帕金森病运动障碍的进展提供安全有效的治疗方法。
https://orcid.org/0000-0002-8364-0219 (Chun-Feng Liu); https://orcid.org/0000-0002-5308-7213 (Deqin Geng)