脑损伤
-
Figure 1| In situ astrocyte-to-neuron direct reprogramming via PTBP1 knockdown.
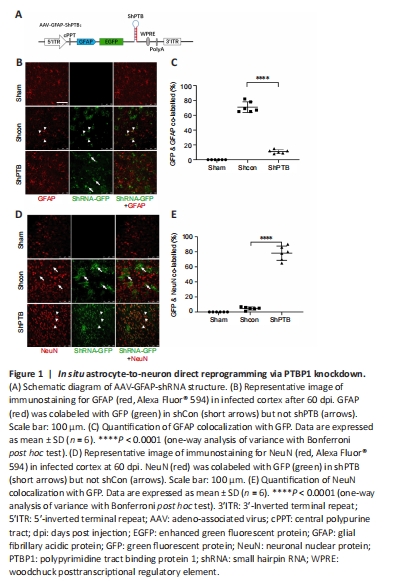
We constructed a set of AAV-PHP.eB viral vectors (Chan et al., 2017) with GFAP promoter-driven miR30-based shRNA (Chang et al., 2013), which were mainly expressed in astrocytes (Figure 1A). Then, PBS, AAV-shCon and AAV-shPTB were injected into the cortex of three mice for 60 days to observe whether infected astrocytes were converted to neurons. No green fluorescent protein (GFP) expression was observed in the sham group, and the shCon and shPTB groups had similar GFP expression, indicating viral infection. Most GFP-positive cells in the shCon group were colabeled with GFAP (approximately 70%; Figure 1B and C) and few overlapped with NeuN (Figure 1D and E), whereas most GFP-positive cells in the shPTB group were colabeled with NeuN (approximately 78%; Figure 1B and C) and few overlapped with GFAP (Figure 1D and E). These data indicated that the shPTB-infected astrocytes were converted to neurons whereas shCon-infected cells retained astrocyte characteristics, indicating that in situ astrocyte-to-neuron direct reprogramming was achieved via PTBP1 knockdown.
Figure 2| Establishment of an ischemic stroke model in FVB mice.
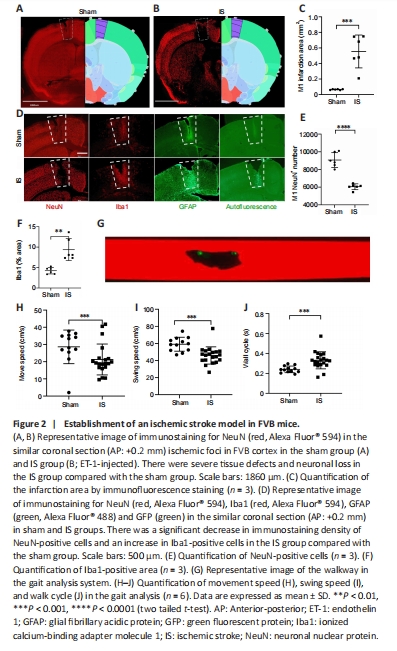
After validating the AAV-PHP.eB-GFAP-shPTB effect, which indicated astrocyte-to-neuron reprogramming in the mouse brain, we examined the effect of direct reprogramming in an ischemic stroke model. Initially, we established an ischemic stroke model in FVB mice by ET-1 injection. At 9 dpi, NeuN immunostaining showed that there were severe tissue defects and neuronal loss in the ischemic stroke group (ET-1 injection) compared with the sham (PBS injection) group (Figure 2A–E). We next stained the tissue with microglia-specific markers Iba1 and GFAP. Compared with those in the sham group, the optical density of staining in microglia and astrocytes were increased around the ischemic foci in the ischemic stroke group (Figure 2D). The above results suggested that there was neuronal loss and gliosis in the ischemic foci after ET-1 injection. Compared with those in the sham group, the number of NeuN-positive cells decreased significantly (P < 0.01; Figure 2E) and Iba1-positive cells increased significantly (P < 0.01; Figure 2F) in the ischemic stroke group. To observe whether behavior was affected in the ischemic model, a gait analysis system (Figure 2G) was used to measure the motor coordination of the mice at 9 dpi. Compared with those in the sham group, the overall movement speed was reduced (P < 0.001; Figure 2H), the swing speed of the left forelimb was reduced (P < 0.0001; Figure 2I), and the walk cycle was prolonged (P < 0.001; Figure 2J) in the ischemic stroke group, indicating that there was a motor deficiency in the model.
Figure 3| Transdifferentiated neurons from shPTB-mediated in situ reprogramming in an ischemic mouse model.

Next, we examined the effect of direct reprogramming in the mouse model of ischemic stroke. The experimental design for brain histology and animal behavior testing is shown in Figure 3A. There was no GFP expression in the sham group injected with PBS, whereas the shCon and shPTB groups showed obvious green fluorescence, indicating AAV infection (Figure 3B and C). We next investigated the time course of shPTB-mediated astrocyte-to-neuron reprogramming around the injected stroke foci. Most GFP-positive cells were colabeled with GFAP in both the shCon and shPTB groups (Figure 3B and D) and few were colabeled with NeuN (Figure 3C and E) around the ischemic foci within 40 dpi. However at 60 dpi, 47% of the GFP-positive cells in the shPTB group were colabeled with NeuN (Figure 3C–E) and fewer were colabeled with GFAP (Figure 3B and D), whereas the GFP-positive cells in the shCon group were colabeled with GFAP (Figure 3B and D) and few were colabeled with NeuN (Figure 3C and E). These data indicated that the AAV-shPTB infected astrocytes were reprogrammed to neurons at around 60 dpi in the mouse model of ischemic stroke.
Figure 4|Characteristics of neurons from shPTB-mediated in situ reprogramming in ischemic foci at 60 dpi.
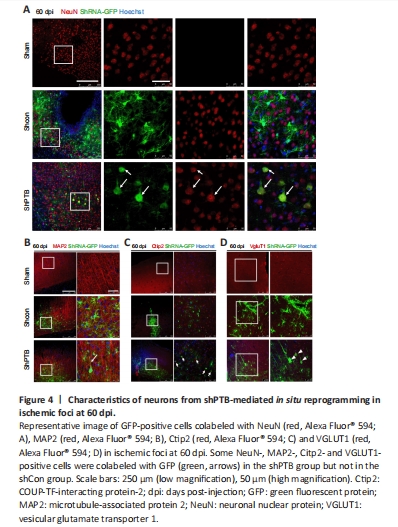
The previous experiment at 60 dpi (Figure 1) suggested that many GFP-positive astrocytes were reprogrammed to neurons in the shPTB group; therefore, 60 dpi was used for further experiments. To confirm the neuronal characteristics of the converted cells from the shPTB-mediated astrocyte reprogramming, immunostaining with the neuronal markers NeuN and MAP2 was performed. A proportion of GFP-positive cell bodies were round with polar structure and were colabeled with NeuN in the shPTB group (Figure 4A). The 3D reconstruction imaging further supported the colocalization of GFP and NeuN (Additional Video 1 and Additional Figure 1A). In contrast, the GFP-positive cells in the shCon group were mainly colabeled with GFAP, and few were colabeled with NeuN (Figure 4A). The GFP-positive cells in the shPTB group were also colabeled with MAP2-stained nerve fibers, which is consistent with neuronal axons, whereas GFP-positive cells were not colabeled with MAP2 in the shCon group (Figure 4B). These results suggested that GFP-positive cells in the shPTB group had neuronal characteristics, with NeuN-positive neuronal nuclei and MAP2-positive nerve fibers, but those in the shCon group did not. To further explore the subtypes of the GFP-positive cells, the cortical neuronal marker Ctip2 and a specific marker of glutamatergic neurons (vesicular glutamate transporter 1, VGLUT1; Chen et al., 2020) were applied. GFP-positive cells in the shPTB group were colabeled with Ctip2 (Figure 4C), which was localized in the nucleus, suggesting that the cells had the characteristic of neurons in deep cortical layers (layers V–VI; Chen et al., 2020). Additionally, GFP-positive cells were colabeled with VGLUT1 in the shPTB group (Figure 4D). The colocalization of GFP and VGLUT1 was further supported by 3D reconstruction imaging (Additional Video 2 and Additional Figure 1B). These data indicated that the shPTB-mediated reprogrammed neurons had characteristics of neurons in deep cortical layers and glutamatergic neurons.
Figure 5|Vascular remodeling and reactive microglia in ischemic foci after in situ reprogramming.
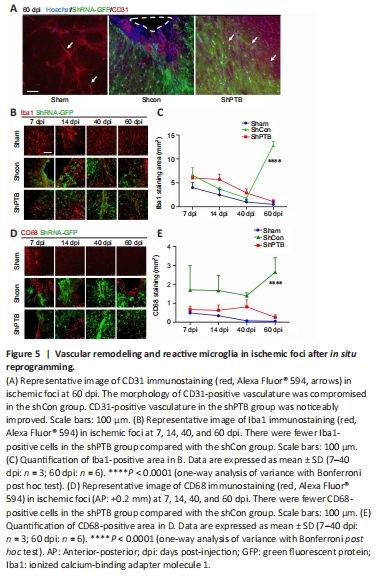
Brain vasculature and vascular remodeling play a critical role in stroke regeneration (Liu et al., 2014). To observe the vascular changes around the ischemic foci after in situ reprogramming at 60 dpi, immunostaining with a vascular endothelial cell marker, CD31, was performed. CD31-positive tissue had clear luminal structures in the sham group (Figure 5A). The morphology of CD31-positive vasculature was compromised and luminal structures were lost in the shCon group. Compared with that in the shCon group, the CD31-positive tissue around the ischemic foci in the shPTB group had a noticeable improvement in the lumina, suggesting that the compromised blood vessels were restored and remodeled in the shPTB group.
Reactive glial cells are a pathological hallmark of neuronal injury (Kiaie et al., 2022). To observe the effect of reactive glial cells around the ischemic foci with shPTB in situ reprogramming, Iba1 immunofluorescence was performed. The Iba1-positive area decreased over time in the sham group (7 dpi vs. 60 dpi, P < 0.05) and shPTB group (7 dpi vs. 60 dpi, P < 0.001), whereas the Iba1-positive area in the shCon group was significantly increased at 60 dpi compared with the previous time points (Figure 5B and C). Similar to the Iba1 staining pattern, the number of cells stained with CD68, an activated microglia marker (lysosomal marker in phagocytes; Hopperton et al., 2018), showed a downward trend in the sham group and shPTB group and was increased in the shCon group. There was a significant increase (P < 0.0001) at 60 dpi in the shCon group compared with that in the sham and shPTB groups (Figure 5D and E), indicating that the shPTB treatment significantly reduced the microglial reaction.
Figure 6|Brain tissue repair in ischemic foci after in situ reprogramming.
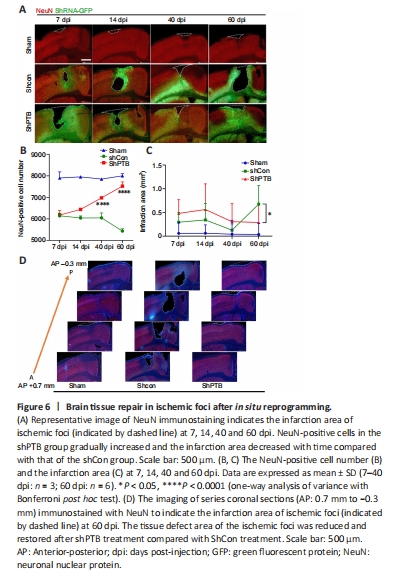
We focused on the ischemic injury core to observe neuronal recovery after viral injection. The ischemic injury core (approximately AP 0.2 mm) was observed under a low-power microscope. In the shCon group, the number of NeuN-positive neurons gradually decreased and the infarction area increased with time. In the shPTB group, the number of NeuN-positive cells gradually increased and the infarction area decreased with time (Figure 6A–C). Notably, there was a significant increase in the number of NeuN-positive neurons and infarction area in the shPTB group compared with those in the shCon group at 60 dpi. Immunofluorescent imaging of the infarction area from the series slides (AP 0.7 mm to ?0.3 mm) demonstrated that the tissue defect area of the ischemic foci was reduced and restored after shPTB treatment (Figure 6D). These data suggested that shPTB-mediated in situ astrocyte-to-neuron reprogramming contributed to neuronal regeneration and brain tissue repair in the stroke model.