脑损伤
-
Figure 1|Change in the hypothalamo–neurohypophyseal system of the hypothalamic pituitary stalk electrical lesion model.

We established a hypothalamic-PEL model using a 3D-printed knife combined with electrolytic injury, and by adopting a transcranial parietal approach, which did not interfere with the anterior pituitary or pituitary portal system. Hypothalamic injury was verified after the procedure using low-magnification microscopy. The results confirmed that the procedure had little effect on other areas of the ventral hypothalamus (Figure 1B). Post-operatively, AVP neurons showed a decrease in nerve fibers and neuronal number over time (Figure 1C). At 10 days post-PEL, the mice showed a massive loss of axons in the median eminence (Figure 1D). Trend analysis demonstrated the significance of the JAK–STAT pathway in rats (Additional Figure 1A–C). Electron microscopy in rats showed that 1 week after the pituitary stalk was destroyed, increased vesicles appeared in neuronal cell bodies (Additional Figure 1D). Moreover, fragments per kilobase million (FPKM) of AVP and OXT also showed a decreasing trend post-PEL (Additional Figure 1E and F).
Figure 3|Normal condition of growth hormone-reactive nucleus and cell types.
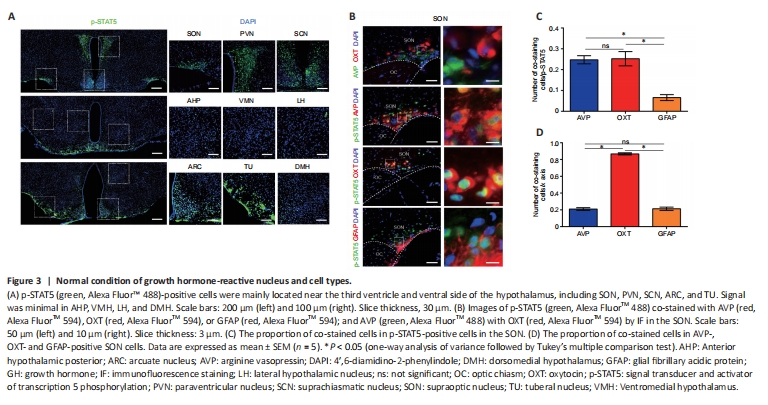
In sham-operated mice, p-STAT5 was highly expressed in the hypothalamus, mainly in the periventricular nucleus and nuclei in the base of the skull, such as the SON, PVN, suprachiasmatic nucleus, tuberal nucleus, and arcuate nucleus (Figure 3A). In the SON, p-STAT5-positive cells were mainly OXT neurons (25.23%), AVP neurons (24.73%), and some astrocytes (6.57%) (Figure 3B–D). In the PVN, p-STAT5-positive cells were mainly OXT neurons (16.61%), with only a small percentage of AVP neurons (3.94%) and some astrocytes (2.76%) (Additional Figure 2A–C). Moreover, 65.25% of OXT neurons in the PVN were p-STAT5 positive, and 86.7% of OXT neurons in the SON were p-STAT5 positive; few AVP neurons and astrocytes were p-STAT5 positive (Figure 3D and Additional Figure 2C), indicating the importance of GH for OXT. In addition, RNA sequencing in rats showed that a large number of GH-related genes were upregulated at 1 day post-PEL and downregulated by 7 days post-PEL based on KEGG pathway analysis (Additional Figure 2D–H).
Figure 4|Expression pattern of p-STAT5 post-pituitary stalk electrical lesion in the supraoptic nucleus.
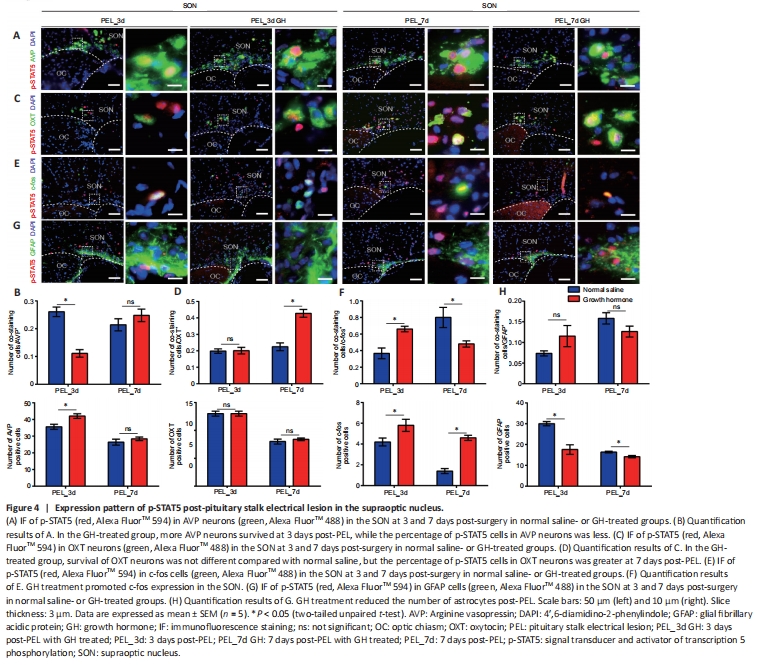
After GH treatment, the number of AVP-surviving neurons did not increase significantly in the SON or PVN by 7 days post-PEL. However, the proportion of p-STAT5-positive AVP neurons was decreased as early as 3 days post-PEL in the SON, suggesting that GH may not have a direct effect on AVP neurons (Figure 4A and B). In addition, more AVP neurons survived in the PVN at 3 days post-PEL and more p-STAT5-positive AVP neurons survived by 7 days post-PEL (8.31% to 37.86%; Additional Figure 3A and B). In the SON, the number of OXT neurons did not increase either with GH treatment post-PEL, but the proportion of p-STAT5-positive cells was increased at 7 days post-PEL (Figure 4C and D). This suggests that GH mainly acts on OXT neurons, while in the PVN, slightly more OXT neurons survived by 7 days post-PEL with GH treatment (Additional Figure 3C and D). In total, more p-STAT5-positive magnocellular neurons survived after GH treatment. In the SON, AVP neurons tended to spread ventrally, while OXT neurons tended to spread dorsally. In the PVN, AVP and OXT neurons tended to show an intermediate distribution. It seems that OXT neurons somehow support AVP neurons. As previously reported, neuronal activation promotes survival after axonal injury (Zhang et al., 2019). Therefore, we detected nuclear expression of c-fos, a protein expressed in active neurons (Zhang et al., 2021), and found an increased portion of c-fos positivity in the GH-treated group (Figure 4E, F and Additional Figure 3E, F). Unfortunately, GH treatment only modestly promoted the survival of AVP and OXT neurons. Considering that astrocytes, as nutritive cells in the CNS, are highly associated with IGF-1 expression (Chesik and De Keyser, 2010), we investigated the GH responsiveness of astrocytes. The number of astrocytes in the GH-treated group was greater at 3 days and less at 7 days post-PEL than in the untreated group, although treatment with GH did not change the increasing trend over time. The number of p-STAT5-positive astrocytes in the GH-treated group decreased, which might be associated with inhibition of inflammation by suppression of astrocyte migration (Figure 4G, H, and Additional Figure 3G, H).
Figure 5|Insulin-like growth factor 1 may act in an autocrine or paracrine manner.
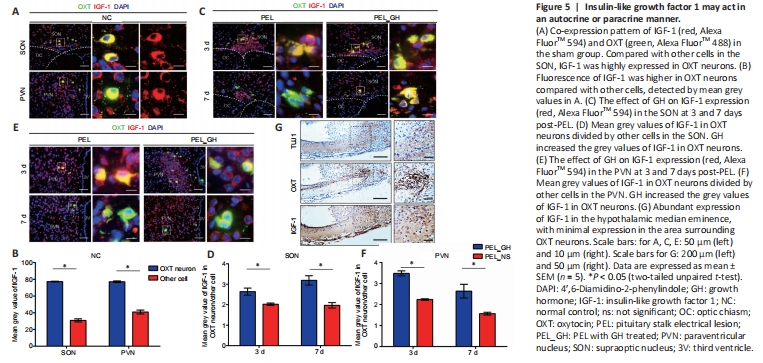
As GH mainly promotes the expression of IGF-1 (Bianchi et al., 2017; Ranke and Wit, 2018), we next examined the expression pattern of IGF-1. In OXT neurons, the fluorescence intensity of IGF-1 was significantly higher than in other cells, suggesting that OXT neurons produce IGF-1 in large quantities (Figure 5A and B). This potential IGF-1 secreting ability of OXT neurons was improved with GH treatment (Figure 5C–F). In addition, the distribution of OXT in nerve fibers of the median eminence did not correlate with IGF-1 (Figure 5G). This suggests that OXT neurons mainly release IGF-1 by autocrine or paracrine signaling through the cell body instead of secreting IGF-1 from axons (Pristerà et al., 2019). AVP did not co-stain with IGF-1 in the sham-operated group, and co-stained cells in the SON were mainly distributed on the dorsal side (Additional Figure 4A and B), indicating that AVP neurons mainly obtain nutrition from other cells. Only a few astrocytes were positive with IGF-1 (Additional Figure 4C). Altogether, these results show that OXT neurons mainly release IGF-1 from the SON and PVN.
Figure 6|Expression of insulin-like growth factor 1 receptor post-pituitary stalk electrical lesion in the supraoptic nucleus.
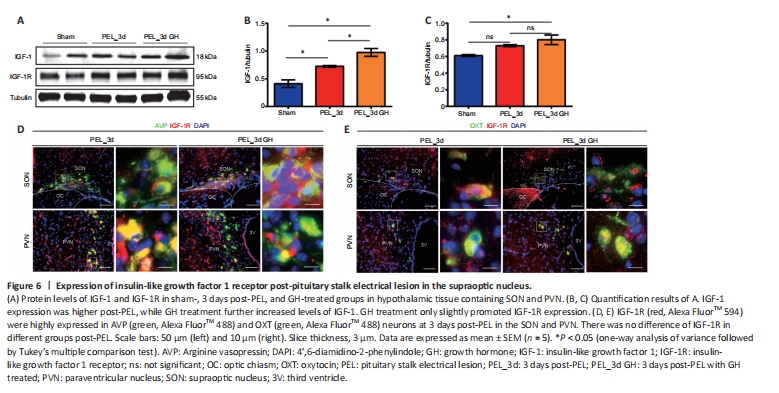
The expression of IGF-1 was upregulated in the nucleus after injury, as shown by western blotting, and the expression of IGF-1 was further promoted by GH treatment (Figure 6A). At the same time, western blot results also suggested an upregulation of IGF-1 receptor (IGF-1R) expression in the nucleus after injury, but GH treatment did not appear to lead to a significant change in the total amount. Notably, GH treatment promoted the retention of IGF-1R at the axonal end of neurons (Additional Figure 5A and B). At 3 days post-surgery, GH did not significantly change IGF-1R expression in AVP and OXT neurons (Figure 6B and C) but seemed to promote IGF-1R expression in the longer-term, as seen at 7 days (Additional Figure 5C). Lastly, the results of RNA-seq in rats suggested that RNA expression of Igf1r was upregulated at 1 day post-PEL and downregulated at 7 days post-PEL, while Igf2r did not change markedly (Additional Figure 5D and E).
Figure 7|Growth hormone treatment promoted axon regeneration.
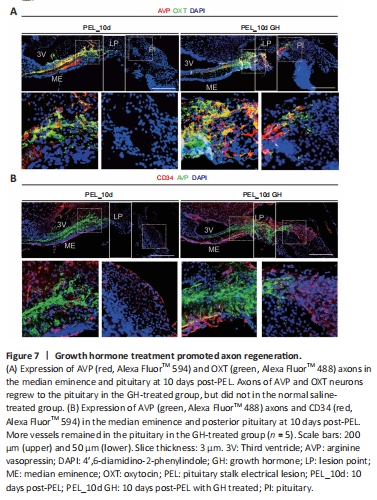
At 10 days post-PEL, axons in the GH-treated group could regenerate back to the posterior pituitary across the lesioned end, whereas axons in the non-GH-treated group could not cross the lesion area (Figure 7A). In addition, axon end vascularization was better with GH treatment, thereby providing a better environment for hormone release, whereas the non-GH treatment group showed atrophy of the posterior pituitary vascular network (Figure 7B). After 10 days of GH treatment, AVP neurons mostly co-stained with OXT neurons in the PVN (Additional Figure 6A), indicating that GH treatment changes the pattern of AVP and OXT expression in magnocellular neurons. In addition, within the area of regenerated axons, we observed β-amyloid expression, which was mainly distributed on the surface of nerve fibers promoting neurite outgrowth (Zhou et al., 2012). Furthermore, GH treatment promoted the retention of glial fibrillary acidic protein-positive cells in the median eminence (Additional Figure 6B). Lastly, PEL mainly injured the hypothalamo-hypophyseal system in the hypothalamus, while merely injured the pituitary (Additional Figure 7).