中国神经再生研究(英文版) ›› 2023, Vol. 18 ›› Issue (7): 1570-1577.doi: 10.4103/1673-5374.360277
L和T型Ca2+通道对实验性青光眼视网膜神经节细胞损伤有迥异作用
L- and T-type Ca2+ channels dichotomously contribute to retinal ganglion cell injury in experimental glaucoma
Hong-Ning Wang1, Wen-Jing Qian1, Guo-Li Zhao1, Fang Li1, Yan-Ying Miao1, Bo Lei2, Xing-Huai Sun3, *, Zhong-Feng Wang1, *
- 1State Key Laboratory of Medical Neurobiology and MOE Frontiers Center for Brain Science, Institutes of Brain Science, Fudan University, Shanghai, China; 2Institutes of Neuroscience and Third Affiliated Hospital, Henan Provincial People’s Hospital, Henan Eye Institute, Henan Eye Hospital, People’s Hospital of Zhengzhou University, Zhengzhou University, Zhengzhou, Henan Province, China; 3Eye Institute and Department of Ophthalmology, Eye & ENT Hospital, NHC Key Laboratory of Myopia, Shanghai Key Laboratory of Visual Impairment and Restoration, Fudan University, Shanghai, China
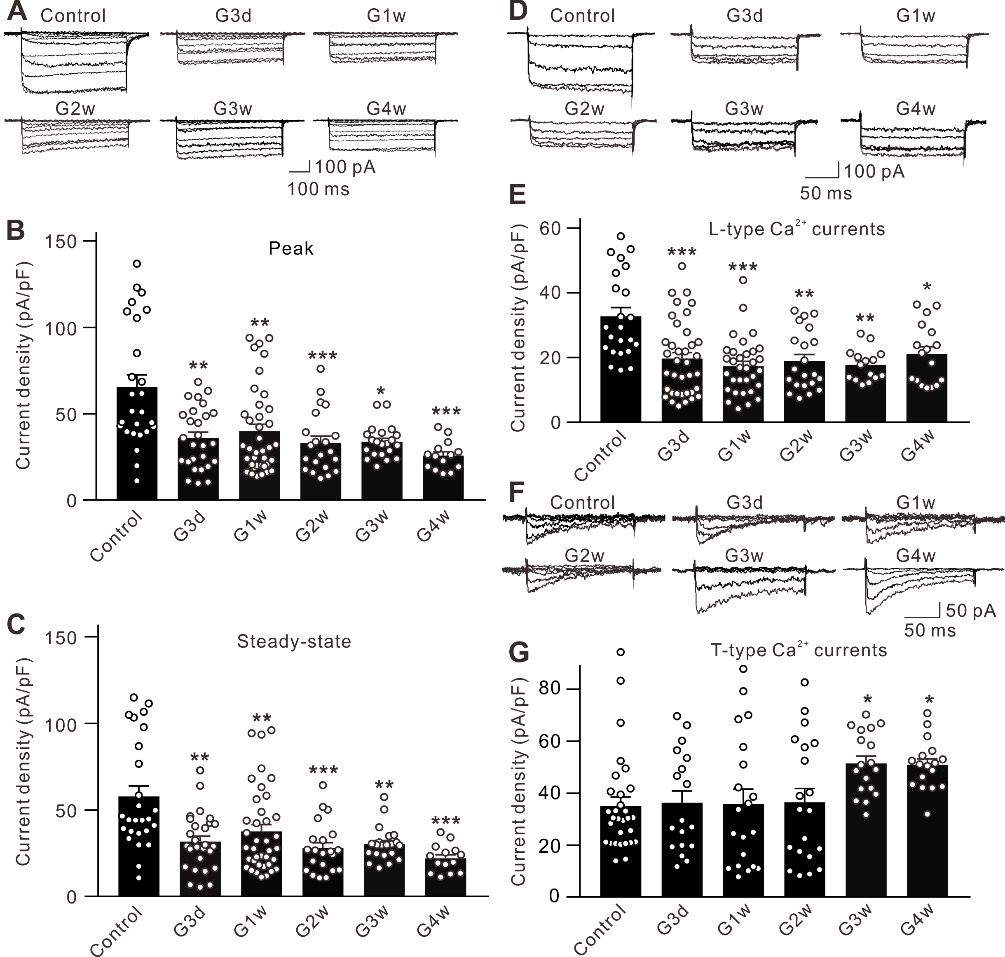
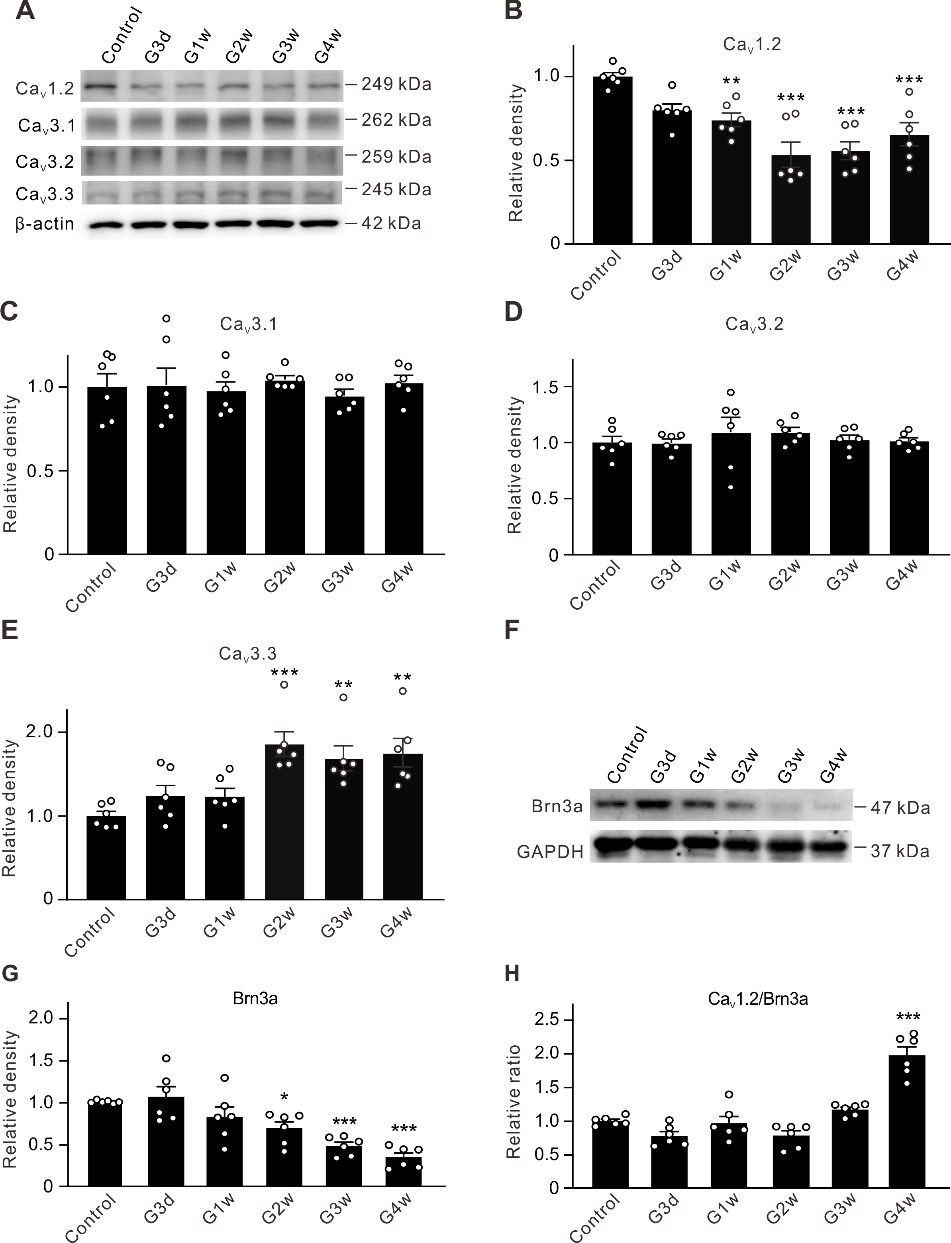
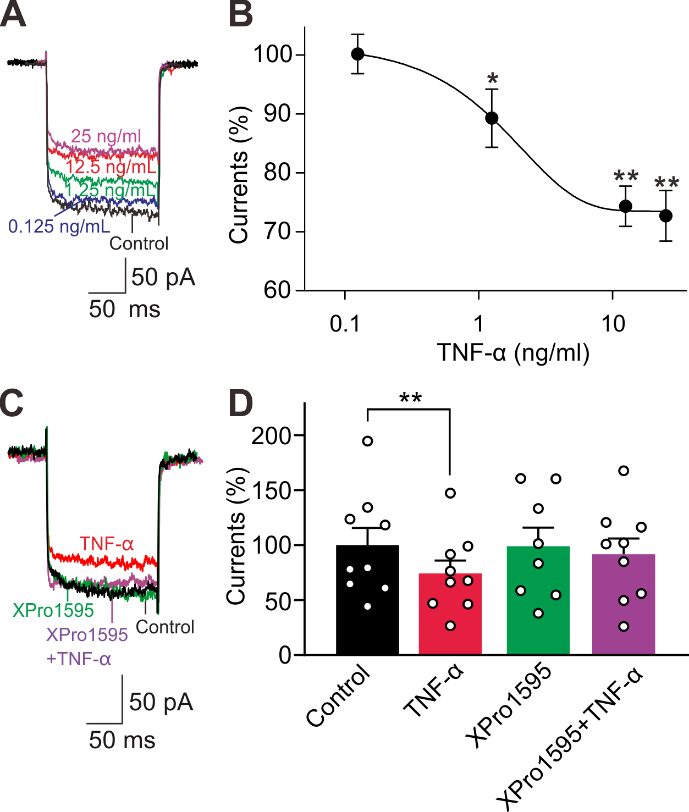
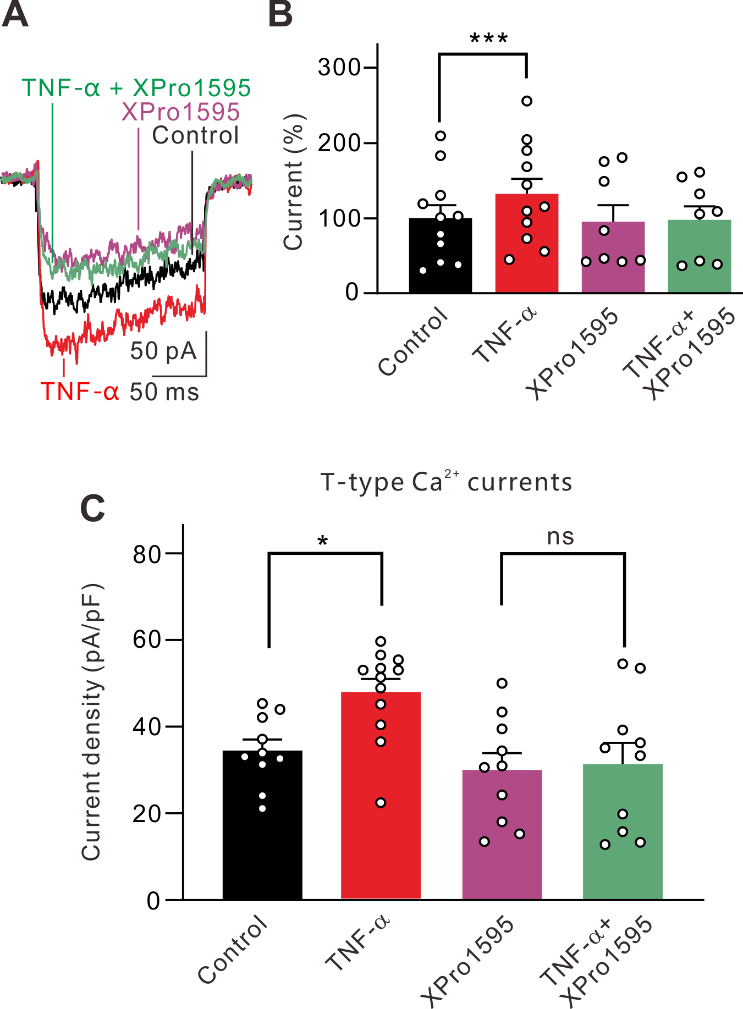
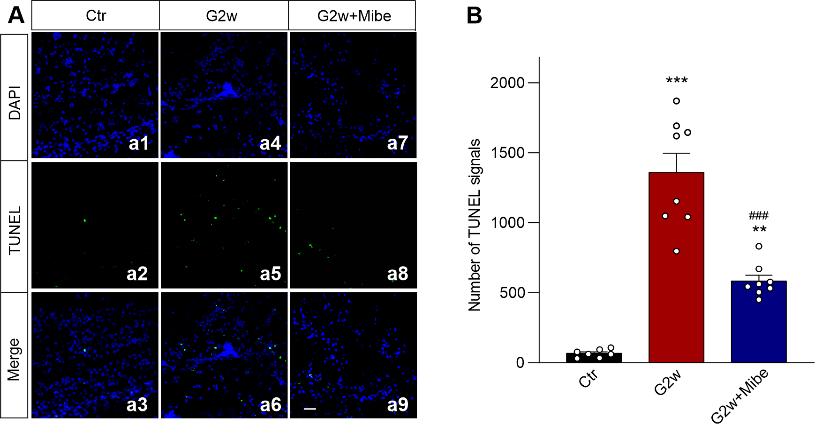
摘要: 视网膜神经节细胞凋亡是导致青光眼不可逆失明的主要病理基础。Ca2+稳态紊乱在青光眼中起重要作用。尽管既往已有研究表明电压门控钙通道阻滞剂可改善青光眼患者的视力,但其是否以及如何参与视网膜神经节细胞凋亡在很大程度上是未知的。作者此次实验利用前房注射微磁珠方法建立了慢性高眼压实验性青光眼大鼠模型,以全细胞膜片钳电生理记录检测发现慢性高眼压大鼠视网膜神经节细胞中的总钙电流密度显著下降,且其中L型钙通道电流密度同步下降;而T型钙通道电流密度明显增加。进一步Western blot和免疫荧光实验可见慢性高眼压视网膜神经节细胞中L型钙通道CaV1.2亚基表达减少,而T型钙通道CaV3.3亚基表达增加。由于炎症因子可溶性肿瘤坏死因子α能抑制分离的正常视网膜神经节细胞L型钙电流,而增强T型钙电流;且上述变化可被肿瘤坏死因子α 抑制剂 XPro1595所阻断,因此这2种Ca2+电流的变化均可由肿瘤坏死因子α介导。而后可见细胞内有丝分裂原活化蛋白激酶/细胞外信号调节激酶通路阻断剂U0126和核因子κB 信号通路阻断剂PDTC可介导肿瘤坏死因子α效应。 最后以TUNEL染色检测视网膜神经节细胞凋亡情况,发现T型钙通道阻断剂米贝拉地尔可显著减少慢性高眼压大鼠模型中凋亡视网膜神经节细胞的数量。
表明T型钙通道可导致青光眼中Ca2+稳态紊乱及视网膜神经节细胞凋亡,而选择性应用T型钙通道阻断剂,特别是CaV3.3特异性阻断剂,可能是临床治疗青光眼的有效策略。
https://orcid.org/0000-0002-2279-6885 (Zhong-Feng Wang); https://orcid.org/0000-0002-4440-1389 (Xing-Huai Sun)