NRR︱南通大学王友华团队揭示MIF调控星形胶质细胞趋化因子产生的机制
撰文:王友华,张涵
脊髓损伤往往引起患者感觉,运动和自主神经功能的丧失[1],且难以恢复[2]。脊髓损伤炎症微环境是影响脊髓功能修复的重要因素[3]。作为关键促炎因子,巨噬细胞迁移抑制因子(Macrophage migration inhibitory factor,MIF)在脊髓损伤炎症微环境的形成中起着重要作用[4-5]。MIF除了直接促发炎症细胞的炎症反应外,是否发挥对炎症细胞的趋化作用尚不清楚。脊髓损伤后,趋化因子CC趋化因子配体2(chemokine C-C motif chemokine ligand 2,CCL2)能够诱导多种免疫细胞在损伤部位的聚集,特别是巨噬细胞和小胶质细胞,从而加剧炎症反应[6-7]。然而,在脊髓损伤微环境中,MIF是否调控CCL2的产生,间接发挥对炎症细胞的趋化功能值得进一步研究。
近日,南通大学附属医院王友华教授团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Macrophage migration inhibitory factor facilitates astrocytic production of the CCL2 chemokine following spinal cord injury”的研究论文。研究人员发现,脊髓损伤诱导促炎因子MIF的升高,继而诱导星形胶质细胞产生趋化因子CCL2。损伤部位CCL2的表达上调,进一步促进小胶质细胞/巨噬细胞的募集,加剧神经炎症的发生。脊髓损伤后,鞘内注射MIF抑制剂4-IPP,可以减少CCL2的产生以及炎症细胞的聚集,从而改善大鼠的运动功能。该发现为治疗脊髓损伤提供了新靶点。
脊髓损伤后过度激活的炎症反应可导致继发性损伤。损伤诱导的趋化因子在炎症细胞的募集和炎症反应激活过程中,发挥关键作用。实验通过转录组测序,检测了大鼠脊髓损伤后不同时间点趋化因子的表达情况(图1);接着,在脊髓损伤模型中检测了MIF和CCL2的表达,并通过鞘内注射MIF抑制剂4-IPP分析了MIF与CCL2之间的调控关系(图2);原代培养星形胶质细胞,并建立小胶质细胞共培养模型,检测星形胶质细胞产生的CCL2对不同亚型炎症细胞的趋化性(图3);最后,在损伤脊髓中注射MIF抑制剂4-IPP。结果显著减少了炎症细胞的募集和损伤脊髓的空洞大小,并促进大鼠运动功能的恢复(图4)。这些结果表明,脊髓损伤后MIF可通过促进星形胶质细胞CCL2的产生,激活神经炎症反应,影响脊髓损伤修复。MIF抑制剂有助于促进改善损伤脊髓的运动功能。
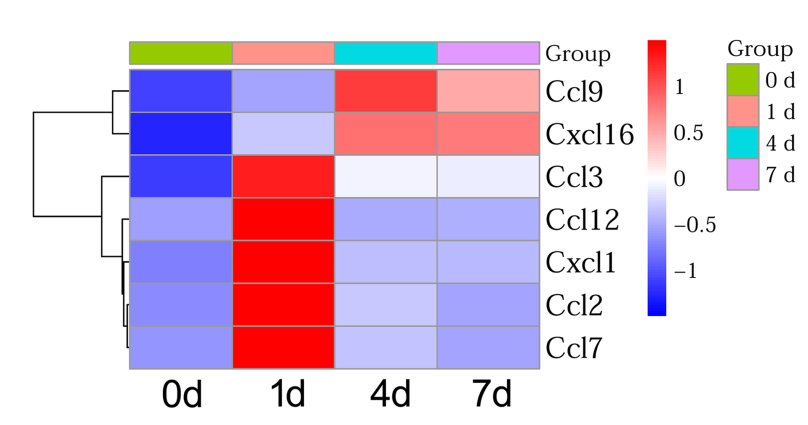 #br#
图1 脊髓损伤后趋化因子表达分析
#br#
图1 脊髓损伤后趋化因子表达分析
(图源:Han Zhang, et al., Neural Regen Res, 2023)
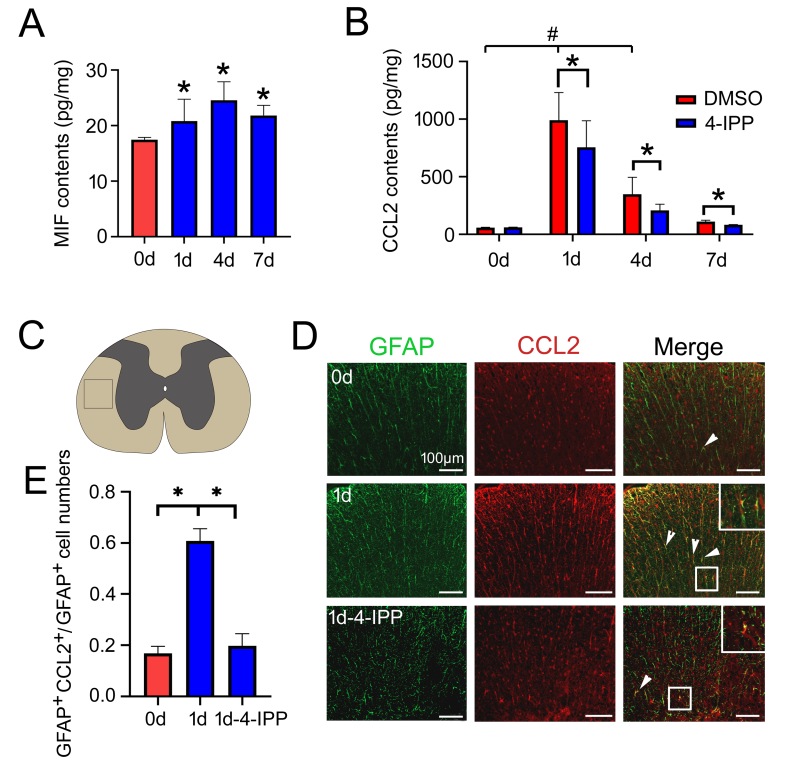 #br#
图2 脊髓损伤后MIF促进星形胶质细胞CCL2的表达
#br#
图2 脊髓损伤后MIF促进星形胶质细胞CCL2的表达
(图源:Han Zhang, et al., Neural Regen Res, 2023)
 #br#
图3 星形胶质细胞产生的CCL2促进不同亚型炎症细胞的趋化。
#br#
图3 星形胶质细胞产生的CCL2促进不同亚型炎症细胞的趋化。
(图源:Han Zhang, et al., Neural Regen Res, 2023)
 #br#
图4大鼠脊髓损伤后注射MIF抑制剂可以促进运动功能恢复#br#
(图源:Han Zhang, et al., Neural Regen Res, 2023)
#br#
图4大鼠脊髓损伤后注射MIF抑制剂可以促进运动功能恢复#br#
(图源:Han Zhang, et al., Neural Regen Res, 2023)
综上所述,脊髓损伤诱导产生的MIF,能够促进星形胶质细胞CCL2的产生,促进炎症反应的进行。损伤早期抑制MIF的活性,可以减少炎症细胞的聚集,从而有利于改善损伤脊髓的运动功能。该研究为脊髓损伤早期临床干预,提供了新的治疗靶点[7- 8]。此外,该研究仍然需要对CCL2或其受体抑制剂,在干预损伤脊髓的效果等方面加以评估。#br#
张涵和胡玉明为论文共同第一作者,徐华和王友华教授为论文共同通讯作者。#br#
#br#
原文链接:https://doi.org/10.4103/1673-5374.363184#br#
#br#
References#br#
[1] Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017; 3:17018.#br#
[2] Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018; 98:881-917.#br#
[3] Anjum A, Yazid MD, Fauzi Daud M, et al. (2020) Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 21:7533#br#
[4] Leyton-Jaimes MF, Kahn J, Israelson A. Macrophage migration inhibitory factor: A multifaceted cytokine implicated in multiple neurological diseases. Exp Neurol. 2018; 301(Pt B):83-91.#br#
[5] Nasiri E, Sankowski R, Dietrich H, et al. Key role of MIF-related neuroinflammation in neurodegeneration and cognitive impairment in Alzheimer's disease. Mol Med. 2020; 26:34.#br#
[6] Jaerve A, Muller HW. Chemokines in CNS injury and repair. Cell Tissue Res. 2012;349(1):229-248.#br#
[7] McTigue DM, Tani M, Krivacic K, et al. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J Neurosci Res. 1998;53(3):368-376.#br#
[8] Dhaiban S, Al-Ani M, Elemam NM, et al. Targeting Chemokines and Chemokine Receptors in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J Inflamm Res. 2020;13:619-633.
 #br#
图1 脊髓损伤后趋化因子表达分析
#br#
图1 脊髓损伤后趋化因子表达分析
 #br#
图2 脊髓损伤后MIF促进星形胶质细胞CCL2的表达
#br#
图2 脊髓损伤后MIF促进星形胶质细胞CCL2的表达
 #br#
#br#
 #br#
图4大鼠脊髓损伤后注射MIF抑制剂可以促进运动功能恢复#br#
(图源:Han Zhang, et al., Neural Regen Res, 2023)
#br#
图4大鼠脊髓损伤后注射MIF抑制剂可以促进运动功能恢复#br#
(图源:Han Zhang, et al., Neural Regen Res, 2023)