中国神经再生研究(英文版) ›› 2023, Vol. 18 ›› Issue (11): 2406-2412.doi: 10.4103/1673-5374.371376
间充质干细胞来源细胞外囊泡修复创伤性中枢神经系统损伤:系统综述和meta分析
Mesenchymal stem cell-derived extracellular vesicles therapy in traumatic central nervous system diseases: a systematic review and meta-analysis#br#
Zhelun Yang#, Zeyan Liang#, Jian Rao, Fabin Lin, Yike Lin, Xiongjie Xu, Chunhua Wang*, Chunmei Chen*
- Department of Neurosurgery, Fujian Medical University Union Hospital, Fuzhou, Fujian Province, China
摘要:
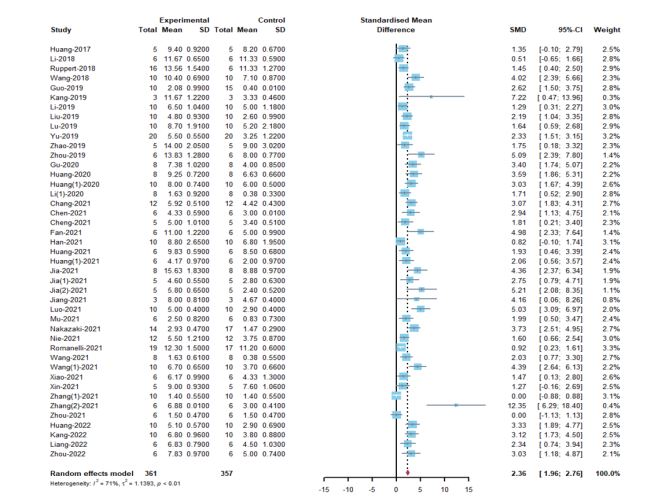
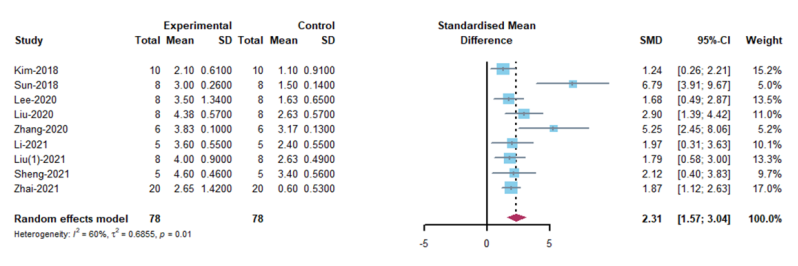
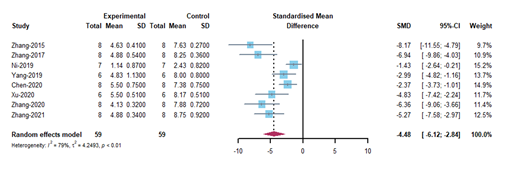
间充质干细胞来源的细胞外囊泡在治疗创伤性中枢神经系统损伤方面已被证明是一种有前途的非细胞疗法。然而,临床前的研究成果要向临床转化需要全方位和系统地掌握相关实验方法和了解间充质干细胞来源的细胞外囊泡对创伤性中枢神经系统损伤的疗效。因此,对近期使用间充质干细胞来源的细胞外囊泡治疗创伤性中枢神经系统损伤的动物试验进行了系统综述和meta分析。通过检索PubMed,Web of Science,The Cochrane Library和Ovid-Embase数据库截止2022年4月1日的数据,筛选间充质干细胞来源的细胞外囊泡治疗创伤性中枢神经系统疾病的临床前研究。应用SYRCLE的偏倚风险工具分析动物研究中的发表偏倚风险。最终60项研究被纳入研究,其中脊髓损伤研究52项,创伤性脑损伤研究8项。结果显示,与对照组相比,间充质干细胞来源的细胞外囊泡治疗可明显促进SCI动物的运动功能恢复,包括大鼠Basso Beattie Bresnahan评分(SMD: 2.36, 95% CI: 1.96-2.76, I² = 71%, P < 0.01)和小鼠BMS评分(SMD: 2.31, 95% CI: 1.57 - 3.04, I² = 60%, P = 0.01);可明显促进创伤性脑损伤动物神经功能的恢复,包括mNSS(SMD: - 4.48, 95% CI: - 6.12 to - 2.84, P < 0.01, I² = 79%)和Foot Fault Test(SMD: - 3.26, 95% CI: - 4.09 to - 2.42, I² = 21%, P = 0.28)。亚组分析显示,对于BBB评分而言,同种异体间充质干细胞来源的细胞外囊泡的疗效高于异种异体间充质干细胞来源的细胞外囊泡(同种异体: SMD: 2.54, 95% CI: 2.05 - 3.02, I² = 65.5%; 异种异体: SMD: 1.78, 95% CI: 1.1 - 2.45, I² = 74.6%; P = 0.0116);通过超滤离心联合密度梯度超速离心分离的间充质干细胞来源的细胞外囊泡(SMD:3.58,95%CI:2.62 - 4.53,I² = 31%,P < 0.0001)可能更有效。对于BMS评分而言,胎盘间充质干细胞来源的细胞外囊泡比骨髓来源的效果更好(胎盘:SMD: 5.25,95%CI:2.45 - 8.06,I² = 0%;骨髓:SMD: 1.82, 95% CI: 1.23 - 2.41, I² = 0%; P = 0.0421)。对于mNSS评分而言,骨髓间充质干细胞来源的细胞外囊泡比脂肪来源的效果更好(骨髓:SMD:-4.86,95%CI:-6.66-3.06,I²=81%;脂肪:SMD:-2.37,95%CI:-3.73-1.01,I²=0%;P=0.0306)。静脉给药(SMD: -5.47, 95% CI: -6.98 - -3.97, I² = 53.3%, P = 0.0002)和给药剂量等于100μg(SMD: -5.47, 95% CI: -6.98 - -3.97, I² = 53.3%, P < 0.0001)显示出更好的结果。各项研究的异质性很小,敏感性分析也表明结果稳定。最后,所有实验的方法学质量大多令人满意。总之,间充质干细胞来源的细胞外囊泡在促进创伤性中枢神经系统损伤后运动和神经功能恢复方面发挥了关键作用。
https://orcid.org/0000-0002-4490-0380 (Chunmei Chen); https://orcid.org/0000-0002-4483-7465 (Chunhua Wang)

 #br#
#br#