NRR: 空军军医大学第一附属医院罗鹏、李新和蒋晓帆团队揭示NMDAR1是重复性轻度创伤性脑损伤的潜在干预靶点
撰文:田志成
创伤性脑损伤的定义是由外部攻击引起的脑损伤,是全球成年人死亡和残疾的主要原因[1-2]。其中,约80%为轻度创伤性脑损伤(mild traumatic brain injury, mTBI)[3]。 由于受伤后症状轻微且短时间会恢复,mTBI经常被忽视。然而,部分mTBI,尤其是重复性mTBI(repetitive mTBI, rmTBI),会发展成为慢性病程,导致神经变性,最终引起功能障碍,如认知功能损害[4-5]。认知功能的改变被认为是与海马结构损伤密切相关[6]。因此,阐明rmTBI后海马脑区蛋白的变化对于进一步揭示rmTBI导致认知障碍的发生具有重要的意义。蛋白组学和磷酸化组学被广泛用于神经系统疾病的研究,来揭示其蛋白变化的规律[7-9]。然而,对于rmTBI后海马脑区的整体蛋白改变,目前仍缺乏研究。N-甲基-D-天冬氨酸受体(N-methyl-D-aspartate receptor, NMDA)在rmTBI中已有研究,主要集中在其亚基NMDAR2[10-14],对于NMDAR1的研究仍缺乏。
近期,空军军医大学第一附属医院罗鹏、李新和蒋晓帆团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表题为“Quantitative proteomic and phosphoproteomic analyses of the hippocampus reveal the involvement of NMDAR1 signaling in repetitive mild traumatic brain injury”的文章。研究发现,rmTBI后海马脑区受损,小鼠产生认知障碍,通过组学深入发现参与认知障碍发生的关键分子NMDAR1,其磷酸化水平升高。进一步证实,抑制海马的NMDAR1能够改善rmTBI导致的认知障碍。这一分子机制为治疗和干预rmTBI提供了潜在靶点,丰富了rmTBI导致认知障碍的分子机制。田志成、曹紫萱和杨二万为论文第一作者,罗鹏、李新和蒋晓帆教授为通讯作者。
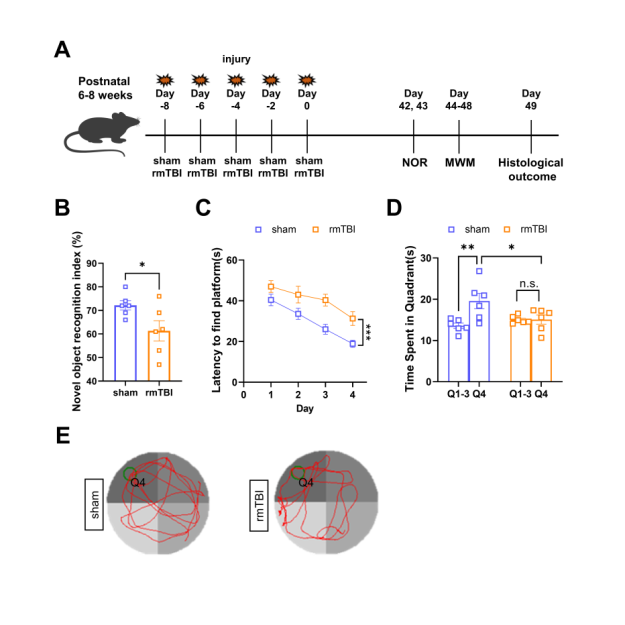
为了探究rmTBI导致认知障碍的具体分子机制,作者首先对小鼠进行了rmTBI模型的建立。模型建立6周后,对小鼠进行了新物体识别实验(Novel object recognition test, NOR)和莫瑞斯水迷宫实验(Morris water maze test, MWM)的测试,结果显示:rmTBI模型导致小鼠NOR指数显著下降;MWM前4天训练阶段,发现平台的潜伏期显著延长;第五天测试阶段,在平台第4象限游泳时间显著缩短。这表明rmTBI导致小鼠产生了认知障碍(图1)。
 #br#
#br#
图1 rmTBI导致海马体迟发的神经功能障碍。(图源:Tian et al., Neural Regen Res, 2023)
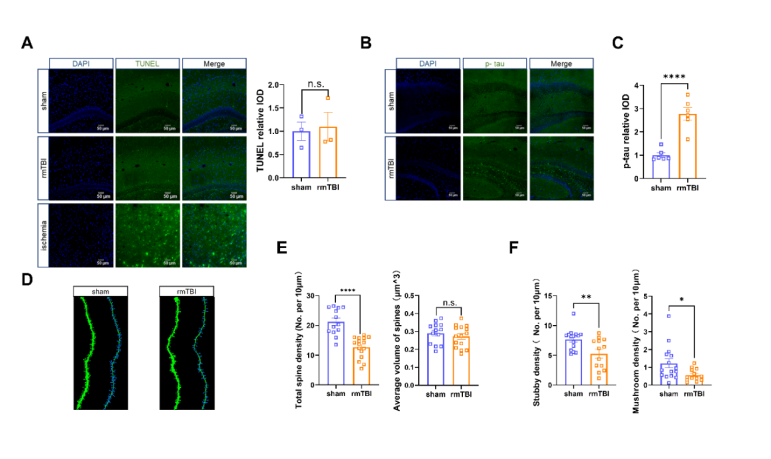
由于海马脑区被认为与认知功能密切相关,因此,为了验证海马脑区是否受损,作者对rmTBI后小鼠的海马进行了细胞凋亡染色(TUNEL染色)和磷酸化tau蛋白(phosphorylation tau, p-tau)染色。通过定量荧光分析,结果显示,rmTBI没有引起显著的海马神经元凋亡(图2A),但p-tau表达显著升高(图2B和C)。这表明rmTBI导致海马脑区受损。为了进一步揭示海马神经元结构是如何改变的,作者对rmTBI后海马神经元进行了高尔基染色,通过对神经元树突棘总密度以及各类树突棘密度的分析,结果显示,rmTBI导致海马神经元树突棘总密度下降以及成熟性树突棘密度显著下降,包括短粗状(Stubby)和蘑菇状(Mushroom)(图2D, E和F)。这表明rmTBI导致海马神经元结构可塑性下降。
 #br#
#br#
图2 rmTBI导致海马结构的损伤。(图源:Tian et al., Neural Regen Res, 2023)
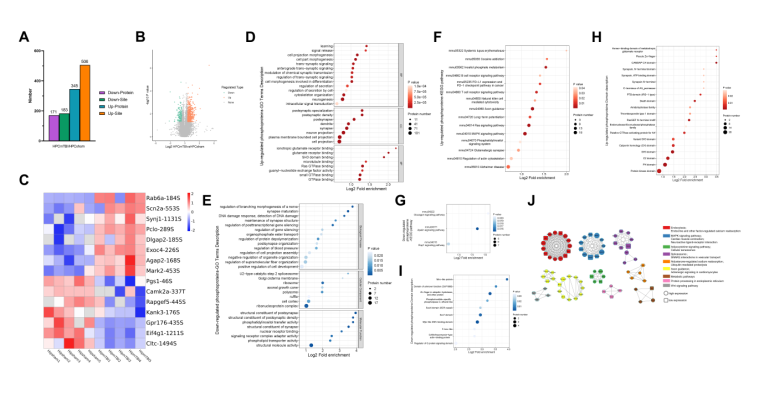
蛋白作为发挥功能的主要执行者,因此,探究rmTBI导致海马蛋白的整体变化特征对于揭示rmTBI导致认知障碍具有重要的意义。作者对rmTBI后6周小鼠的海马脑区进行了蛋白组学和磷酸化组学的研究,对结果进行深入的生信分析,包括GO(Gene Ontology)过程富集、KEGG(Kyoto Encyclopedia of Genes and Genomes)通路富集以及蛋白结构域富集。蛋白组学结果显示,差异表达蛋白(differentially expressed proteins, DEPs)主要富集在炎症、免疫和凝血相关的过程和通路上(图3),这些变化反映了rmTBI最初的变化。这表明rmTBI后炎症和免疫反应可能在海马体中持续存在。磷酸化蛋白组学结果显示,差异表达磷酸化蛋白(Differentially expressed phosphoproteins, DEPPs)的富集过程及通路与神经功能障碍和神经元结构变化导致的神经变性更为一致(图4)。这表明,DEPPs更符合rmTBI模型的生物表型。
 #br#
#br#
图3 海马蛋白组学分析结果。(图源:Tian et al., Neural Regen Res, 2023)
 #br#
#br#
图4 海马磷酸化组学分析结果。(图源:Tian et al., Neural Regen Res, 2023)
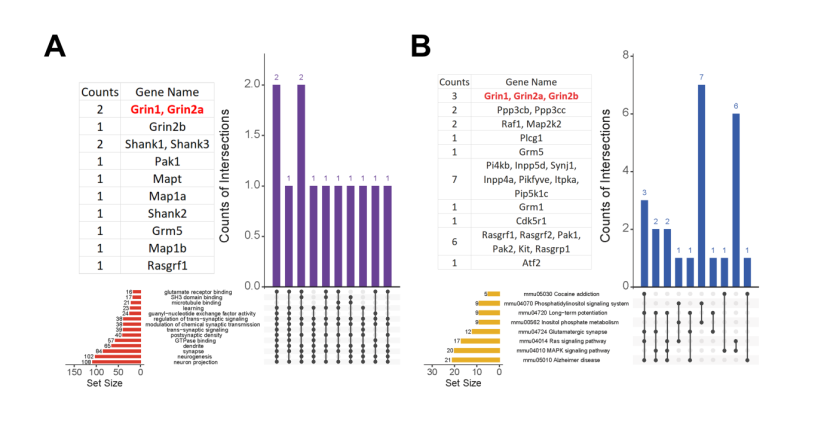
基于rmTBI导致认知障碍及神经元结构损伤,作者对磷酸化组学中与神经元结构和功能相关的GO富集分析结果以及KEGG通路分析结果进行了深入分析。通过绘制Upset图,发现在GO和KEGG中最大交集程度包含2个关键分子:谷氨酸离子型受体NMDA型亚基1(Glutamate Ionotropic Receptor NMDA Type Subunit 1, Grin1)和谷氨酸离子型受体NMDA型亚基2A(Glutamate Ionotropic Receptor NMDA Type Subunit 2a, Grin2a),即NMDAR1和NMDAR2(图5)。
 #br#
#br#
图5 在GO过程和KEGG通路富集分析中的关键分子。(图源:Tian et al., Neural Regen Res, 2023)
既往在rmTBI中,对NMDA的研究主要集中在NMDAR2,还未有关于rmTBI后NMDAR1磷酸化在海马中发挥作用的研究。同时,NMDAR1是NMDA发挥功能的基本亚基,因此,作者研究集中在了NMDAR1。蛋白质免疫印迹结果显示,rmTBI导致海马脑区NMDAR1表达量未发生变化,但其磷酸化水平显著升高,这与组学结果是相一致的(图6A)。
为了验证NMDAR1是否参与rmTBI导致认知障碍发生,作者通过给药管对rmTBI小鼠海马脑区特异性给予NMDAR1拮抗剂CGP78608。给药后30分钟,对小鼠进行NOR测试,结果显示,CGP78608导致rmTBI小鼠的NOR指数升高,这表明抑制NMDAR1可以改善rmTBI导致的认知障碍(图6C)。
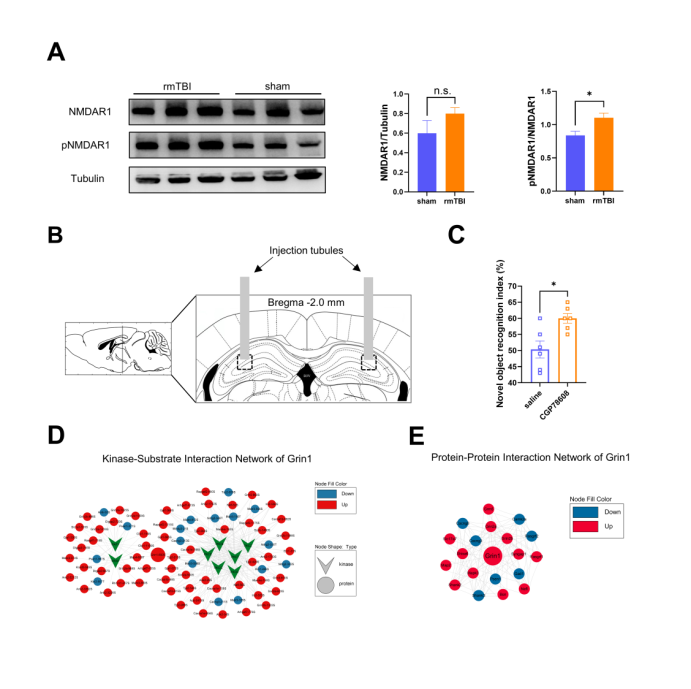 #br#
#br#
图6 海马中的NMDAR1参与调节rmTBI后认知障碍的发生。(图源:Tian et al., Neural Regen Res, 2023)
总而言之,该研究表明rmTBI导致海马脑区结构受损,小鼠产生认知障碍,海马的NMDAR1磷酸化水平显著升高。通过抑制海马的NMDAR1能够改善rmTBI导致的认知障碍。该研究揭示了NMDAR1信号转导是rmTBI导致慢性认知障碍的机制之一,可能是干预和治疗rmTBI的潜在靶点。
该研究也存在着一定的局限性。首先,该研究只关注了海马脑区,在大脑中,可能还有其他脑区参与rmTBI后认知障碍的发生,这些区域可能构成神经环路。未来,通过对不同脑区进行研究可能揭示rmTBI后独特的发病机制,特别是与生物学功能相关的分子机制,这有助于更好的认识该疾病。另外,该研究在不同蛋白质的分辨率和细胞特异性方面具有局限性,主要研究神经元的变化。神经元和神经胶质细胞在神经系统中各自发挥着功能,分别研究它们在rmTBI后的变化,有助于实施有针对性地干预措施。最后,该研究对NMDAR1在rmTBI中作用的研究仅进行了初步地验证,其具体调控机制仍未阐明。因此,对于rmTBI的发病机制仍需要更多的研究进行充分且全面地揭示。
原文链接:
参考文献:
[1] Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. Recent advances in traumatic brain injury. J Neurol. 2019;266:2878-2889.
[2] Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104:213-238.
[3] Heyburn L, Sajja V, Long JB. The role of tdp-43 in military-relevant tbi and chronic neurodegeneration. Front Neurol. 2019;10:680.
[4] Gao X, Chen J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J Neuropathol Exp Neurol. 2011;70:183-191.
[5] Gilmore CS, Lim KO, Garvin MK, et al. Association of optical coherence tomography with longitudinal neurodegeneration in veterans with chronic mild traumatic brain injury. JAMA Netw Open. 2020;3:e2030824.
[6] Castellano JM, Mosher KI, Abbey RJ, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544:488-492.
[7] Liao L, Mcclatchy DB, Yates JR. Shotgun proteomics in neuroscience. Neuron. 2009;63:12-26.
[8] Hosp F, Mann M. A primer on concepts and applications of proteomics in neuroscience. Neuron. 2017;96:558-571.
[9] Bharucha T, Gangadharan B, Kumar A, et al. Mass spectrometry-based proteomic techniques to identify cerebrospinal fluid biomarkers for diagnosing suspected central nervous system infections. A systematic review. J Infect. 2019;79:407-418.
[10] Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in n-methyl-d-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128:305-322.
[11] Giza CC, Maria NS, Hovda DA. N-methyl-d-aspartate receptor subunit changes after traumatic injury to the developing brain. J Neurotrauma. 2006;23:950-961.
[12] Park Y, Luo T, Zhang F, et al. Downregulation of src-kinase and glutamate-receptor phosphorylation after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1642-1649.
[13] Mei Z, Qiu J, Alcon S, et al. Memantine improves outcomes after repetitive traumatic brain injury. Behav Brain Res. 2018;340:195-204.
[14] Carvajal FJ, Cerpa W. Regulation of phosphorylated state of nmda receptor by step(61) phosphatase after mild-traumatic brain injury: role of oxidative stress. Antioxidants. 2021;10:1575.
作者介绍:
第一作者:田志成(空军军医大学第一附属医院神经外科,博士在读)、曹紫萱(空军军医大学基础医学院,本科在读)和杨二万(空军军医大学第一附属医院神经外科,硕士在读)
通讯作者:罗鹏(空军军医大学第一附属医院神经外科,副教授)、李新(空军军医大学第一附属医院麻醉科,副教授)和蒋晓帆(空军军医大学第一附属医院神经外科主任,教授)
 #br#
#br#
 #br#
#br#
 #br#
#br#
 #br#
#br#
 #br#
#br#
 #br#
#br#