中国神经再生研究(英文版) ›› 2024, Vol. 19 ›› Issue (7): 1593-1601.doi: 10.4103/1673-5374.385839
神经干细胞源性外泌体促进阿尔茨海默病线粒体生发和重塑其脑区化分布异常
Neural stem cell-derived exosomes promote mitochondrial biogenesis and restore abnormal protein distribution in a mouse model of Alzheimer’s disease
Bo Li1, #, Yujie Chen2, #, Yan Zhou1, #, Xuanran Feng3, Guojun Gu1, Shuang Han2, Nianhao Cheng2, Yawen Sun1, Yiming Zhang1, Jiahui Cheng1, Qi Zhang4, *, Wei Zhang1, *, Jianhui Liu3, *#br#
- 1Department of Radiology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China; 2Morphology and Spatial Multi-Omics Technology Platform, Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, Shanghai, China; 3Department of Anesthesiology, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China; 4Department of Blood Transfusion, Huashan Hospital, Fudan University, Shanghai, China
摘要:
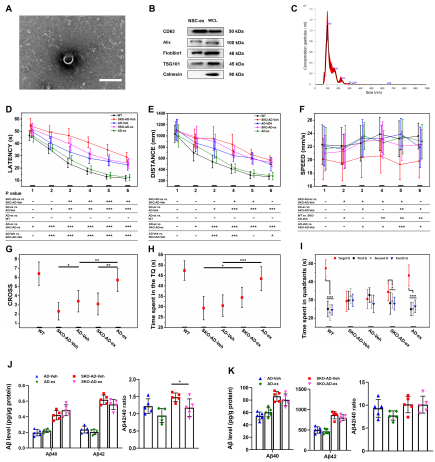
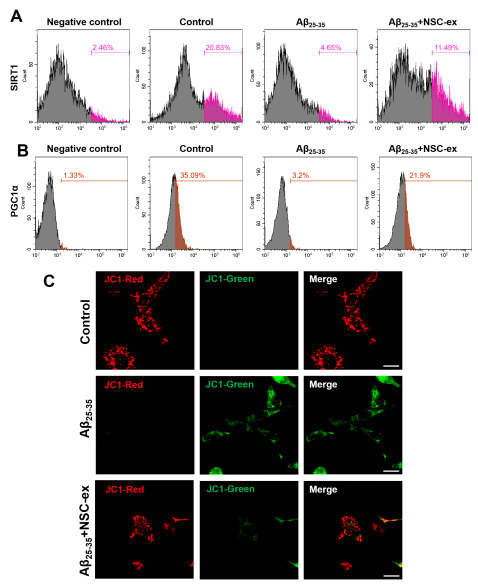
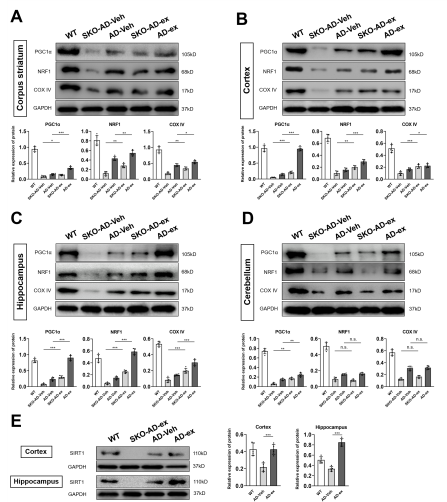
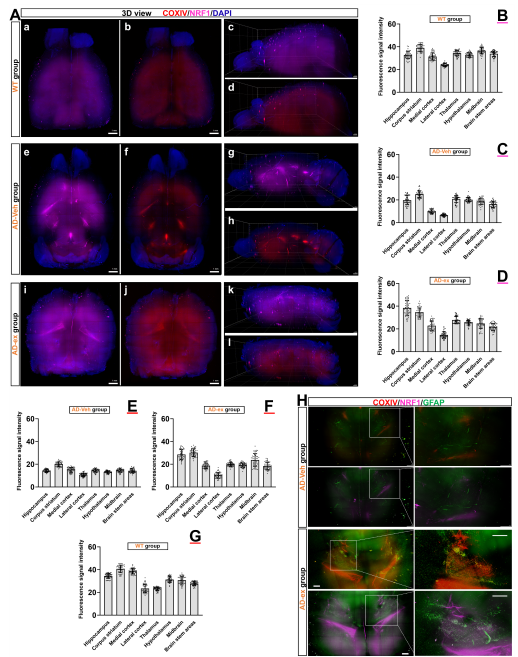
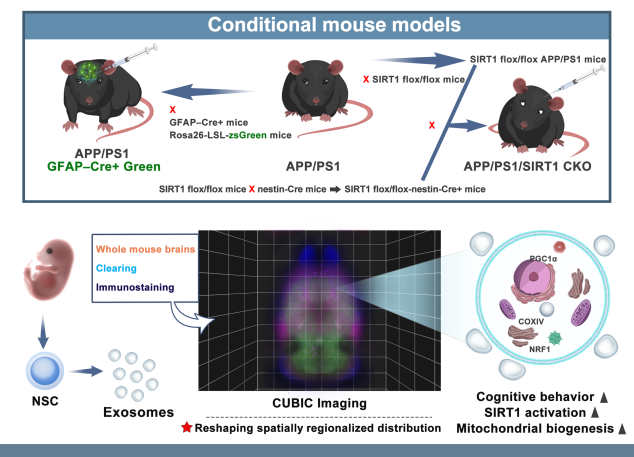
线粒体功能障碍是阿尔茨海默病(AD)的主要特征。既往研究证明小鼠神经干细胞源性外泌体(NSC-ex)对阿尔茨海默病(AD)有潜在治疗作用并改善了小鼠大脑皮层的线粒体功能。考虑到AD是影响全脑功能状态的疾病,在前期相关研究的基础上,实验进一步研究了NSC-ex疗法对多脑区线粒体生发的影响以及运用全脑透明化成像技术分析了治疗前后线粒体生发状态的改变,以探究NSC-ex对于AD可能的潜在治疗靶点。结果显示,NSC-ex可以提高AD小鼠SIRT1水平,增强线粒体功能,重塑线粒体生发相关重要蛋白PGC1α,NRF1和COXIV的无序空间脑区化分布,减少星形胶质细胞的激活,最终改善认知功能。通过对比Aβ25-35和新型神经系统特异性sirtuin 1(SIRT1)条件性敲除APP/PS1小鼠模型,发现SIRT1基因敲除AD小鼠行为表现更差,多脑区的线粒体生发相关因子水平更低,Aβ水平更高,证明SIRT1基因缺失引起更严重的病理进展和认知障碍。此外,在3种重塑线粒体生发相关重要蛋白中,NSC-ex治疗对于PGC1α的改善更为明显,而NRF1和COXIV的恢复表现有一定相似性。然而,NSC-ex干预只对Aβ水平有轻微的积极作用。总体而言,这项研究结果强调了NSC-ex治疗对AD引起的线粒体生发相关蛋白脑区化异常分布的整体重塑,并提示线粒体功能促进可能是NSC-ex发挥功能的一个重要靶点,在这其间SIRT1-PGC1α相关通路可能发挥了重要作用。
https://orcid.org/0000-0002-7330-1811 (Qi Zhang); https://orcid.org/0000-0001-9766-5495 (Wei Zhang); https://orcid.org/0000-0002-8335-0078 (Jianhui Liu)

 #br#
#br#

