NRR:湘雅医院宋伟涛团队揭示p38 MAPK抑制剂治疗青光眼的作用机制
青光眼是世界首位不可逆性致盲性眼病,预计到2040年全球将有1.118亿人患有青光眼。(1)视网膜神经节细胞 (Retinal ganglion cells, RGCs) 的选择性、进行性死亡是所有类型青光眼的最终结局。(2)几种类型的细胞死亡,如细胞凋亡、坏死、自噬和细胞焦亡等均被认为与青光眼RGCs损失相关。而基于这些死亡方式提出的潜在药物在临床上并未能有效地发挥RGC保护作用。这提示研究者需要对RGCs的死亡有新认识。谷氨酸兴奋性神经毒性已被证实在青光眼中发挥重要作用。(3)且在既往研究中,谷氨酸被证实可以诱导铁死亡。(4)铁死亡是一种新型的细胞死亡方式,其特征是铁依赖性脂质过氧化。(5, 6) p38 丝裂原活化蛋白激酶 (MAPK) 通路抑制剂SB202190是有潜力的铁死亡抑制剂,其下游分子P53等,被证实与铁死亡相关。(4, 7)在谷氨酸兴奋性毒性作用下,RGCs中是否也发生了铁死亡,以及抑制铁死亡缓解谷氨酸兴奋性毒性引起的RGCs损失,这些问题尚不明确。
近期,湘雅医院宋伟涛团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“P38 MAPK inhibitor SB202190 preventing ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model”的研究论文。作者发现,谷氨酸兴奋性毒性诱导RGCs发生铁死亡,使用SB202190可以有效抑制铁死亡,同时能一定程度上阻止RGCs损失。进一步研究证实,SB202190的这些作用可能是通过调节铁死亡通路中Gpx-4,SLC7A11,FTL和SAT1实现的。该文带来的启示:抑制铁死亡可能是实现青光眼RGCs保护的有效途径,SB202190是有潜力的视神经保护药物。冯乐蒙和王超为论文共同第一作者,宋伟涛副教授为论文通讯作者。
既往研究发现,谷氨酸可诱导铁死亡。(4)此次研究中,谷氨酸兴奋性毒性引起大鼠视网膜前体细胞中铁离子堆积、脂质过氧化物累积、MDA升高及GSH降低。电子显微镜下观察到谷氨酸作用下,线粒体双层膜结构密度增加、线粒体嵴减少以及线粒体外膜破裂。这些结果均提示,谷氨酸可诱导大鼠视网膜前体细胞铁死亡(图一)。铁死亡受多种信号通路调节,上游分子机制非常复杂。SLC7A11/GSH/GPX4通路是细胞内抵御铁死亡的最主要抗氧化系统。SLC7A11,是胱氨酸/谷氨酸转运蛋白,可将胱氨酸转运至细胞内,并将其氧化成半胱氨酸来催化合成GSH。(8)GSH是GPX-4清除脂质活性氧的必要因子。(9, 10) SLC7A11升高可通过影响GPX4活性抑制铁死亡发生。与先前的报道一致,在宋伟涛等的中发现,SB202190通过调节SLC7A11/GSH/GPX4信号通路减少R28细胞中的脂质ROS积累,抑制铁死亡(图二)。FTL是细胞内铁储存蛋白,该蛋白下调会导致细胞内铁稳态失衡,加速铁死亡发生。(11)本研究中SB202190上调了FTL的表达,降低细胞内游离的二价铁离子,一定程度上抑制了铁死亡。SAT1是多胺代谢的重要调节剂,其活化可诱导脂质过氧化,触发ROS引起的铁死亡。(12, 13)在这里,我们发现SB202190可以有效抑制谷氨酸引起的SAT1高表达。这进一步表明SB202190通过调节脂质过氧化在铁死亡的干预中发挥了一定作用(图三)。既往研究中,谷氨酸兴奋性毒性可引起RGCs减少,和视网膜神经节细胞复合体 (retinal ganglion cell body complex, GCC) 变薄。(14)在该研究中发现,SB202190可以阻止RGCs的损失,GCC变薄以及视网膜功能的丧失(图四)。上述结果说明,SB202190具有缓解视网膜兴奋性毒性的作用,且起作用与抑制铁死亡有关。
总之SB202190是有潜力的青光眼视神经保护因子。通过抑制p38 MAPK,玻璃体腔内注射SB202190可以调节铁死亡相关蛋白,在一定程度上阻止谷氨酸引起的视网膜损伤和视功能丧失。当然该研究也存在一定性局限性。研究仅基于谷氨酸兴奋性毒性模型。尽管这一模型已被广泛应用于青光眼神经保护研究,但单一模型不足以充分模拟临床青光眼患者RGC丢失的病理和生理过程。为了全面分析RGCs的铁死亡和p38 MAPK抑制剂的治疗效果,有必要在未来的研究中纳入更多的青光眼动物模型。

图1 SB202190保护R28细胞系免受谷氨酸诱导的铁死亡
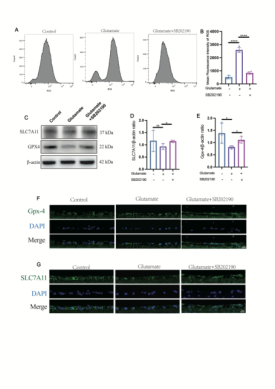
图2 SB202190通过调节slc7a11/gsh/gpx4通路清除胞内脂质活性氧

图3 SB202190通过作用于ftl 和sat1调节谷氨酸R28细胞模型中的铁死亡

图4 SB202190减轻大鼠视网膜中NMDA诱导的损伤
原文链接:https://doi.org/10.4103/1673-5374.391193
参考文献
[1]Kang JM, Tanna AP. Glaucoma. Med Clin North Am. 2021;105:493-510
[2]Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901-1911.
[3]Wang S, Liao L, Huang Y, et al. Pin1 is regulated by CaMKII activation in glutamate-induced retinal neuronal regulated necrosis. Front Cell Neurosci. 2019;13:276.
[4]Li S, Huang Y. Ferroptosis: an iron-dependent cell death form linking metabolism, diseases, immune cell and targeted therapy. Clin Transl Oncol. 2022;24:1-12.
[5]Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072.
[6]Li J, Cao F, Yin HL,et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88.
[7]Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62.
[8]Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599-620.
[9]Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med. 2020;152:175-185.
[10]Zhang Y, Swanda RV, Nie L, et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12:1589.
[11]Bu W, Liu R, Cheung-Lau JC, et al. Ferritin couples iron and fatty acid metabolism. FASEB J. 2012;26:2394-2400
[12]Mou Y, Zhang L, Liu Z, et al. Abundant expression of ferroptosis-related SAT1 is related to unfavorable outcome and immune cell infiltration in low-grade glioma. BMC Cancer. 2022;22:215.
[13]Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162-168.
[14]Liu M, Li H, Yang R, et al. GSK872 and necrostatin-1 protect retinal ganglion cells against necroptosis through inhibition of RIP1/RIP3/MLKL pathway in glutamate-induced retinal excitotoxic model of glaucoma. J Neuroinflammation. 2022;19:262.

通讯作者宋伟涛副教授




