NRR:“铁死亡与少突前体细胞的秘密”:苏州大学附属第一医院虞正权/李海英课题组揭示脑缺血后少突胶质前体细胞铁死亡及髓鞘损伤的发生机制
撰文:虞正权,李海英,杨坚
脑缺血具有高发病率、高死亡率和高致残率的特点[1]。中枢神经系统中存在大量的少突胶质前体细胞,可分化为成熟的髓鞘形成少突胶质细胞以再髓鞘化受损的轴突。少突胶质前体细胞对缺血低氧以及与脑缺血相关的谷氨酸和ATP兴奋毒性的有害影响表现出较高的敏感性,并会走向死亡[2]。这将引起髓鞘的丢失或再生不足,并导致长期神经功能障碍,尤其是脑白质损伤[3, 4]。然而,少突胶质前体细胞死亡在脑缺血后的发生机制尚不清楚。
铁死亡是一种铁依赖性膜脂质过氧化导致细胞膜破裂的细胞死亡形式,在包括脑缺血在内的多种中枢神经系统疾病中发挥了重要的作用[5]。少突胶质前体细胞对铁死亡敏感,它们富含膜多不饱和脂肪酸和铁,并依赖谷胱甘肽抗氧化[6-8]。然而,关于脑缺血后少突胶质前体细胞铁死亡,及在发病和预后中的研究非常少。
转录组测序技术和生信分析手段是研究疾病发生不可缺少的重要工具,有助于阐述疾病发生机制、发现有效治疗靶点。然而,尚无生物信息学分析来探讨脑缺血后少突胶质前体细胞中铁死亡的发生及调控机制。
中国苏州大学附属第一医院的虞正权和李海英课题组在《中国神经再生研究(英文)》 (Neural Regeneration Research) 上发表了题为 “Perilipin-2 mediates ferroptosis in oligodendrocyte progenitor cells and myelin injury after ischemic stroke” 的研究,其阐明了Perilipin-2(PLIN2)介导少突胶质前体细胞铁死亡是脑缺血后髓鞘损伤的重要机制。实验首先对公共数据库中转录组测序数据进行分析,发现脑缺血后少突胶质前体细胞中铁死亡水平上升,少突分化和髓鞘形成水平下降,且Plin2表达升高并与这些过程显著相关。进一步使用小鼠脑缺血再灌注模型发现,脑缺血后可发生髓鞘损伤,胼胝体梗死周围出现少突胶质前体细胞的死亡和脂质过氧化水平的升高,并被铁死亡抑制剂Ferrostatin-1逆转。机制上,PLIN2可能介导了脑缺血后少突胶质前体细胞铁死亡,而敲低Plin2在髓鞘损伤和运动功能恢复中发挥保护作用。该研究提示靶向PLIN2调控少突胶质前体细胞铁死亡可能是脑缺血后髓鞘损伤的潜在治疗策略。
既往针对中枢神经系统中铁死亡的研究,主要集中在神经元,近期在脊髓损伤和脑出血中的研究发现少突胶质前体细胞铁死亡导致脱髓鞘和神经功能损伤[9, 10]。生物信息学分析可以帮助揭示与疾病相关的分子机制,是研究疾病发生的重要方法。因此虞正权和李海英等首先对公共数据库中的转录组测序数据进行生信分析,探索脑缺血后少突胶质前体细胞的功能改变及相关机制。对小鼠白质缺血后第5天分选胼胝体少突胶质前体细胞的批量转录组测序数据进行差异基因分析和基因集富集分析(gene set enrichment analysis, GSEA),结果发现缺血组中包括铁死亡在内的细胞死亡通路活性上升,而包括少突胶质细胞分化、髓鞘形成在内的少突胶质前体细胞功能通路活性下降(图1)。随后进行蛋白互作分析(protein-protein interaction, PPI)和加权基因共表达网络分析(weighted gene co-expression network analysis, WGCNA),分别得到一个关键模块,对模块内的基因进行基因功能富集分析,结果发现模块内的基因与铁死亡、少突胶质细胞分化、髓鞘相关(图1和2)。这些结果表明:白质缺血可能诱导少突胶质前体细胞发生铁死亡。
图1白质缺血分选少突胶质前体细胞的转录组和蛋白互作分析揭示了少突胶质前体细胞铁死亡(图源:Yang et al., Neural Regen Res, 2025)
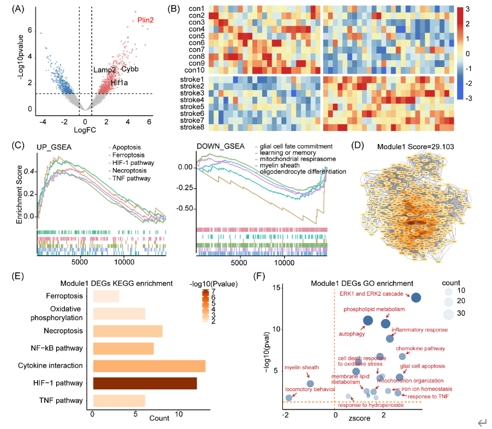
图2加权基因共表达网络分析和关键模块的获取(图源:Yang et al., Neural Regen Res, 2025)
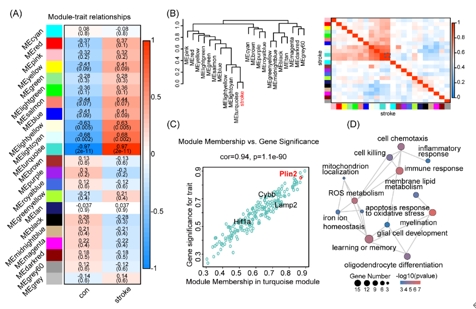
由于少突胶质细胞系存在异质性及铁死亡发生的时空差异,虞正权和李海英等使用小鼠脑缺血再灌注一天的皮层单细胞转录组测序数据对少突胶质细胞进行细分,旨在确定铁死亡敏感亚型,并阐明可能的调控机制。提取少突胶质细胞进行再次降维聚类后得到2个少突胶质前体细胞亚群,将加权基因共表达网络分析得到的铁死亡模块在2个亚群中进行映射,结果发现该模块在脑缺血后的亚群1中显著富集。随后对亚群分别进行功能富集分析,结果发现亚群1中铁死亡和少突胶质前体细胞功能通路显著富集,而在亚群2中没有富集到铁死亡通路。亚群基因表达分析结果显示亚群1中铁死亡发生相关基因的表达水平较高,正调控少突分化、髓鞘化基因的表达水平较低。细胞通讯结果显示亚群1与其他少突胶质细胞间互作降低。基因集富集分析显示亚群1中铁死亡通路活性上升,并得到重要的领头基因亚集。以上分析表明脑缺血后存在铁死亡敏感少突胶质前体细胞群,并出现细胞功能异常。
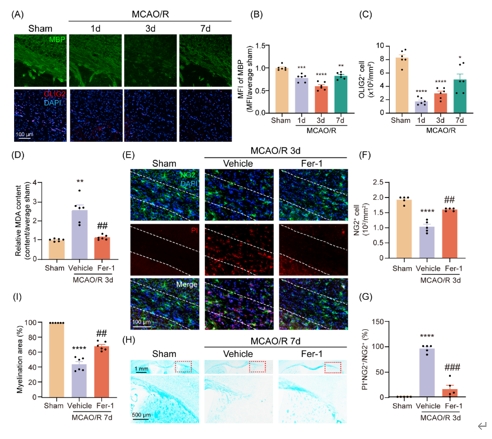
基于以上生信分析结果,虞正权和李海英等使用小鼠脑缺血再灌注模型验证脑缺血后少突胶质前体细胞铁死亡。通过免疫荧光染色发现,脑缺血再灌注1d时髓鞘碱性蛋白荧光强度降低,并伴随少突胶质细胞数量减少,并持续到7d。脂质过氧化产物丙二醛定量结果显示,脑缺血后胼胝体梗死周围组织丙二醛含量上升。利用碘化丙哌啶进行死亡细胞标记,并与少突胶质前体细胞的细胞类型标记物进行免疫荧光共染,结果显示脑缺血后胼胝体梗死周围出现少突胶质前体细胞的死亡。接下来使用牢固蓝染色特异识别髓鞘损伤,结果发现脑缺血后髓鞘发生明显的病理损伤。最后使用铁死亡抑制剂Ferrostatin-1评估挽救少突胶质前体细胞铁死亡在脑缺血后髓鞘损伤修复中的作用,丙二醛测量、碘化丙哌啶染色和牢固蓝染色发现,Ferrostatin-1处理减少了少突胶质前体细胞的死亡,降低了脂质过氧化水平,并改善了髓鞘损伤(图3)。以上证据表明,脑缺血再灌注后发生少突胶质前体细胞铁死亡和髓鞘损伤,伴有脂质过氧化水平的升高,并能被Ferrostatin-1挽救。
图3脑缺血再灌注胼胝体梗死周围发生少突胶质前体细胞铁死亡和脱髓鞘并能被Ferrostatin-1挽救(图源:Yang et al., Neural Regen Res, 2025)
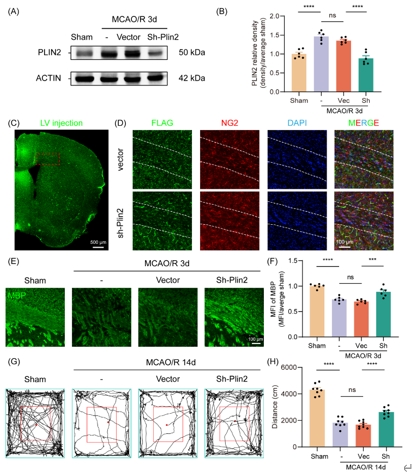
为探索少突胶质前体细胞铁死亡的可能机制,虞正权和李海英等将生信分析得到的蛋白互作分析关键模块、加权基因共表达网络分析关键模块和基因集富集分析领头亚集中的基因取交集,并最终得到1个关键基因:周脂素2(Perilipin 2, PLIN2)。PLIN2参与脂质储存和代谢,是细胞中对缺氧敏感的脂滴积聚标志物,与多种代谢紊乱有关[11]。在批量转录组测序数据中对Plin2表达量与铁死亡、少突胶质前体细胞功能通路活性做相关性分析,结果提示Plin2与脂质过氧化、少突分化、髓鞘形成功能显著相关。通过免疫荧光染色发现脑缺血再灌注 3d 胼胝体梗死周围少突胶质前体细胞中PLIN2水平显著上调(图4)。利用慢病毒敲低Plin2,并进行髓鞘碱性蛋白荧光染色和旷场行为学,发现病毒干预后白质损伤和小鼠运动功能显著改善(图5)。这些结果表明,PLIN2可能参与介导少突胶质前体细胞铁死亡,导致髓鞘损伤,并可能与脂质过氧化相关。
图4脑缺血后PLIN2水平上升可能介导少突胶质前体细胞铁死亡和髓鞘损伤(图源:Yang et al., Neural Regen Res, 2025)
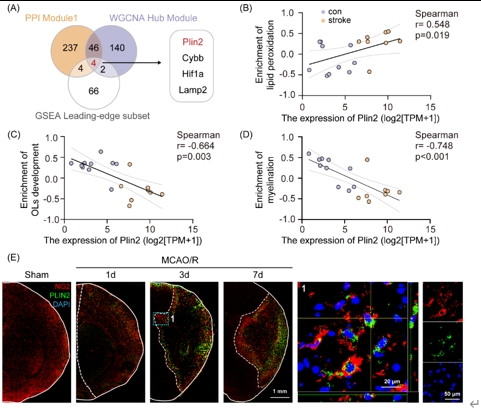
图5 Plin2敲低挽救了脑缺血再灌注后脱髓鞘及小鼠运动功能损伤(图源:Yang et al., Neural Regen Res, 2025)
综上所述,应用转录组测序数据分析了脑缺血后少突胶质前体细胞的功能变化,继而构建了共表达网络,以确定与少突胶质前体细胞铁死亡有关的调控基因。而后利用数据库中的单细胞转录组测序数据分析了少突胶质前体细胞异质性、亚群功能及细胞间互作对脑缺血的反应,最终确定了关键基因并进行了验证。实验还建立了脑缺血/再灌注模型,发现模型小鼠髓鞘损伤明显并伴有少突胶质前体细胞的死亡和脂质过氧化水平的升高,这些效应可被铁死亡抑制剂Ferrostatin-1逆转。脑缺血后少突胶质前体细胞中PLIN2水平显著上升,敲低Plin2挽救了脱髓鞘并改善了小鼠运动功能缺损。此项研究结果对于开发靶向脑缺血后髓鞘损伤的治疗方法具有借鉴意义。
同时,此项研究的生信结果提示少突胶质前体细胞可能存在其他细胞死亡形式,包括坏死和凋亡。细胞死亡间的相互作用决定了脑缺血中受损细胞的结局,继续探索铁死亡是否在决定少突胶质前体细胞的命运中发挥关键作用,铁死亡与其他细胞死亡形式间的交叉作用,以及对各种细胞死亡机制采取干预策略具有必要性。另外,如何促使存活少突胶质前体细胞的增殖、迁移、分化、成熟、髓鞘再生等,都是治疗脑缺血后髓鞘损伤所需关注的问题。
该研究同时存在一些有待解决的问题。此项研究主要集中在4个候选基因中的1个,忽视了对其余基因功能的探索,也没有深入研究基因间的相互作用,会过度简化对复杂生物过程的理解。另外,铁死亡相关少突胶质前体细胞亚群的生物信息学和实验数据缺乏严格验证,削弱了在这一特定亚群中的研究结果的稳健性。此项研究实验验证仅限于蛋白水平,忽略了对转录和翻译水平的研究。最后,PLIN2参与铁死亡的具体机制及其在少突胶质前体细胞功能中的作用仍未得到探讨。
总之,此项研究提示,靶向PLIN2调控少突胶质前体细胞铁死亡可能是脑缺血后髓鞘损伤的潜在治疗策略。
原文链接:https://doi.org/10.4103/NRR.NRR-D-23-01540
参考文献
[1] Saini V, Guada L, Yavagal DR. Global Epidemiology of stroke and access to acute ischemic stroke interventions. Neurology. 2021;97(20 Suppl 2):S6-S16.
[2] Song FE, Huang JL, Lin SH, et al. Roles of NG2-glia in ischemic stroke. CNS Neurosci Ther. 2017;23(7):547-553.
[3] Huang S, Ren C, Luo Y, et al. New insights into the roles of oligodendrocytes regulation in ischemic stroke recovery. Neurobiol Dis. 2023;184:106200.
[4] Zhang C, Guan Q, Shi H, et al. A novel RIP1/RIP3 dual inhibitor promoted OPC survival and myelination in a rat neonatal white matter injury model with hOPC graft. Stem Cell Res Ther. 2021;12(1):462.
[5] Costa I, Barbosa DJ, Benfeito S, et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol Ther. 2023;244:108373.
[6] Spaas J, Van Veggel L, Schepers M, et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol Life Sci. 2021;78(10):4615-4637.
[7] Kilanczyk E, Saraswat Ohri S, Whittemore SR, et al. Antioxidant protection of NADPH-depleted oligodendrocyte precursor cells is dependent on supply of reduced glutathione. ASN Neuro. 2016;8(4):1759091416660404.
[8] Cheli VT, Correale J, Paez PM, et al. Iron metabolism in oligodendrocytes and astrocytes, implications for myelination and remyelination. ASN Neuro. 2020;12:1759091420962681.
[9] Wang Y, Li W, Wang M, et al. Quercetin prevents the ferroptosis of OPCs by inhibiting the Id2/transferrin pathway. Chem Biol Interact. 2023;381:110556.
[10] Wu W, Luo Z, Shen D, et al. IL-10 protects against OPC ferroptosis by regulating lipid reactive oxygen species levels post stroke. Redox Biol. 2024;69:102982.
[11] Adeniyi PA, Gong X, Macgregor E, et al. Ferroptosis of microglia in aging human white matter injury. Ann Neurol. 2023;94(6):1048-1066.




