NRR:中国南京医科大学李柯然团队揭示肠道菌群在年龄相关性黄斑变性中的重要作用
金倩子,王苏豫,姚雨佳,蒋沁,李柯然
年龄相关性黄斑变性是一种视网膜神经退行性疾病,伴有进行性光感受器变性和视网膜色素上皮功能障碍。全球患病率约为8.7%,是发达国家60岁及以上成年人失明的主要原因。由于感光细胞和视网膜色素上皮细胞都不能再生,年龄相关性黄斑变性是一种不可逆的疾病。目前研究发现,年龄相关性黄斑变性的发病机制涉及年龄、环境、遗传易感性等因素的复杂组合,但其具体机制尚不清楚。根据病情进展,年龄相关性黄斑变性可分为多个阶段。早期为干性年龄相关性黄斑变性,也称为黄斑萎缩,其特征是视网膜视网膜色素上皮层和Bruch膜之间有白蛋白沉积。这一阶段伴有视力下降、视力模糊等症状。脉络膜新生血管是晚期年龄相关性黄斑变性的一个重要特征,通常被称为新生血管性年龄相关性黄斑变性 或湿性年龄相关性黄斑变性。在这一时期,由血管内皮生长因子驱动的血管生成可导致光感受器丧失和视力损伤。抗血管内皮生长因子玻璃体内注射是目前治疗湿性年龄相关性黄斑变性的一线治疗方法,但它仅延缓了中枢性视力丧失的发生。更重要的是,干性年龄相关性黄斑变性仍然没有可行的治疗方法,而干性年龄相关性黄斑变性占年龄相关性黄斑变性病例的大多数。最新的《中国老年性黄斑变性诊疗临床指南(2023)》也强调了年龄相关性黄斑变性的早期治疗。因此,有必要研究年龄相关性黄斑变性的病理生理过程,特别是干性年龄相关性黄斑变性,并探索有效的微创治疗方案。从而抑制年龄相关性黄斑变性的发生和发展。
最近,中国南京医科大学李柯然团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“The gut–eye axis: from brain neurodegenerative diseases to age-related macular degeneration”的综述。许多脑神经退行性疾病都强调了肠脑轴的参与,延伸到眼部,许多眼部疾病也受肠道菌群的调控。科技的进步促进了对肠眼轴的理解,为年龄相关性黄斑变性的预防和治疗提供了有前途的途径。未来的研究需要充分了解肠道菌群与年龄相关性黄斑变性之间的关系及其潜在的分子机制。
年龄相关性黄斑变性是一种严重威胁视力的视网膜神经退行性疾病,其具体发病机制仍不清楚,且缺乏早期治疗方法。肠道微生物群是一个与宿主共存的大型生态系统。肠道微生物因年龄、饮食、遗传等因素而发生动态变化。肠道菌群失调可破坏微生态平衡,引起宿主免疫和代谢功能紊乱,影响多种疾病的发生发展。人们认为肠道菌群的生态失衡与肠易激综合征等许多慢性胃肠道疾病密切相关。然而,近些年来,许多证据证明肠道菌群也会影响消化道以外的系统,例如大脑。几项研究表明,肠脑轴在脑神经退行性疾病(包括阿尔茨海默病和帕金森病)的发展中起着关键作用 [1, 2]。与中枢神经系统类似,“肠眼轴”在许多眼部疾病的发病机制中所起的作用已得到证实。此外,年龄相关性黄斑变性和许多脑神经退行性疾病有几个共同的风险因素和相似的病因 [3]。因此,肠道菌群很有可能在年龄相关性黄斑变性中发挥重要作用。
健康成人肠道中有1000多种共生微生物,其中99%为细菌。肠道菌群由大约100万亿个微生物组成,数量是人体细胞的十倍。它们的集体遗传物质包括多达300万个基因,超过人类基因组100多倍。人类的“第二基因组”一词已经成为肠道微生物组的一个描述,或者被称为“宏基因组”,反映了肠道中大量多样的微生物遗传物质。门、纲、目、科、属、种是细菌水平的分类,门是最高的。健康人肠道菌群包含多个门,其中厚壁菌门和拟杆菌门约占总数的90%。其他门包括变形菌门、放线菌门等。在健康个体中,肠道菌群在“有益菌”和“致病菌”之间保持平衡。肠道菌群失调可通过肠眼轴引起各种眼部疾病。目前肠道菌群的研究方法有许多种,包括16S rRNA基因测序、宏基因组测序、转录组测序、代谢组学、器官芯片等。每种方法有各自的优缺点,在未来可以结合多种技术来克服单一方法的局限性,从而深入研究尚未开发的肠眼轴领域,给年龄相关性黄斑变性带来新的见解和治疗策略。
肠道菌群作为人体的重要组成部分,具有多种生理功能,包括食物和药物代谢,并保持肠粘膜屏障的结构完整性。此外,肠道菌群可以促进免疫系统的生长和成熟,保护宿主免受病原体的侵袭。代谢物是肠道菌群影响机体并产生实质性作用的主要机制。肠道菌群的代谢底物主要来源于未消化的食物和内源性分子。大量研究强调,肠道菌群可以通过多种途径与中枢神经系统相互作用,在神经退行性疾病中发挥关键作用,这被称为“肠脑轴”。涉及这一过程多种机制,包括由迷走神经介导的神经通路和携带肠道菌群代谢物和免疫成分的全身循环。此外,肠内分泌细胞分泌的激素有助于肠道和大脑之间复杂的相互作用。肠道菌群组成的改变,也被称为生态失衡,可导致肠道通透性增加和促炎条件的发生。“漏肠”被定义为肠道屏障的崩溃,导致病原体相关的分子模式(如细菌成分、有害代谢物和炎症信号)在血液中不受控制地通过。通过体循环,这些与病原体相关的分子模式可以通过激活众多细胞表达的不同模式识别受体而引起炎症。循环促炎物质水平升高可损害血视网膜屏障和血脑屏障,导致通透性增加和功能障碍,进一步促进神经炎症。
虽然年龄相关性黄斑变性的确切病因尚不清楚,但由于年龄相关性黄斑变性与脑神经退行性疾病有许多相似之处,年龄相关性黄斑变性的发病机制可能受到肠道菌群及其代谢物的显著影响。在生理条件下,肠道菌群在维持体内稳态中起着关键作用。在病理状态下,肠道和眼屏障的破坏促进细菌转运进入血液,从而对眼部组织产生影响。最近有研究调查了年龄相关性黄斑变性患者体内的肠道菌群,使用不同的样品、测序方法和检测到的细菌(表1)。Zinkernagel等 [4]于2017年发起了第一次宏基因组测序调查,发现年龄相关性黄斑变性患者肠道菌群发生了变化。随后(3年后),同一组进行了另一项更大样本量和补体缺乏小鼠的研究 [5],发现湿性年龄相关性黄斑变性患者肠道菌群的改变与之前的研究相似,并且与补体系统密切相关。此外,在孟德尔随机化调查发现了年龄相关性黄斑变性的一些风险和保护因素[6, 7]。一种常用的年龄相关性黄斑变性动物模型是激光诱导脉络膜新生血管形成模型,用氩气激光破坏小鼠眼睛的布鲁氏膜,诱导脉络膜新生血管形成,模拟湿性年龄相关性黄斑变性。一些研究人员还进行了动物实验,发现年龄相关性黄斑变性患者的肠道菌群和代谢物发生了变化 [8-11]。

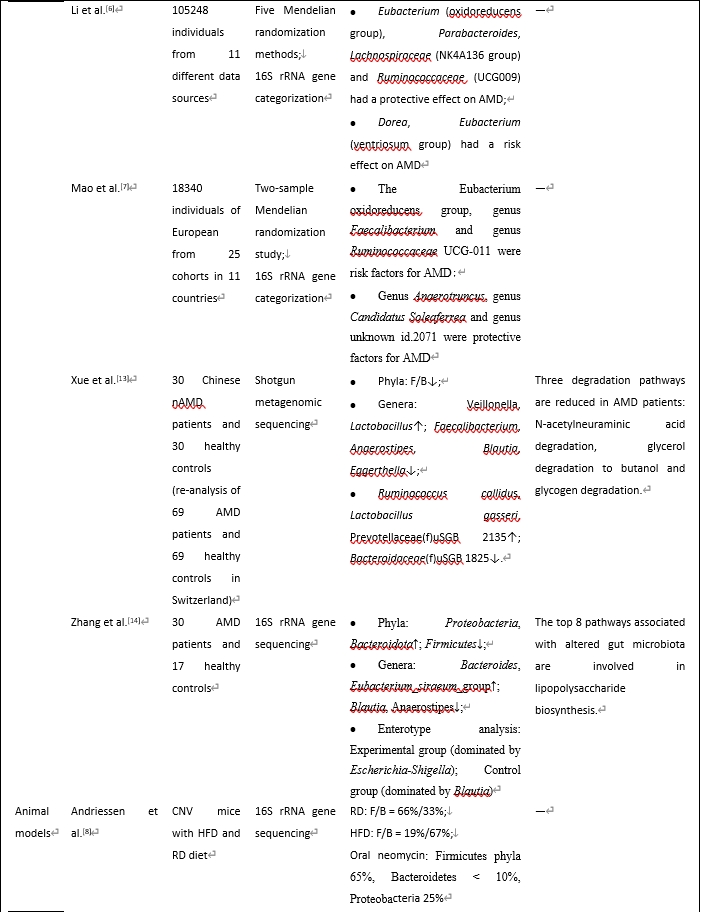
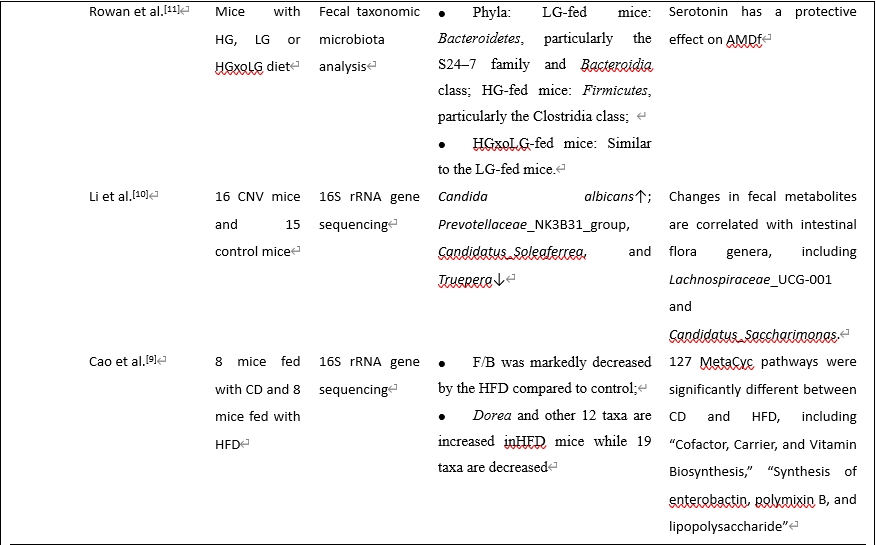
综合这些研究结果,我们发现厚壁菌门/拟杆菌门比值可能是年龄相关性黄斑变性的一个有用的生物标志物。厚壁菌门和拟杆菌门构成了健康人的大部分肠道菌群。这2个“细菌王国”的营养和生存需求相当,然而它们在生态和代谢特性上有所不同。它们在生理和病理状态中的比例也有显著差异。
既往研究表明,肥胖个体和动物模型肠道菌群中的拟杆菌数量减少,而厚壁菌门的丰度显著增加,减肥可以恢复肠道菌群组成。因此,厚壁菌门/拟杆菌门比值升高是肥胖相关肠道生态失调的常见标志。鉴于肥胖与年龄相关性黄斑变性风险增加之间的既定关联,我们假设肥胖可能通过提高肠道菌群中的厚壁菌门/拟杆菌门水平来增加年龄相关性黄斑变性风险。在一项大型全基因组关联研究中发现了年龄相关性黄斑变性的多种遗传变异。调节补体级联的变异与年龄相关性黄斑变性的关系最为显著,包括补体因子H和补体因子I基因。Zysset-Burri等 [5]发现c3缺陷小鼠肠道菌群表现为厚壁菌门增加,拟杆菌门减少。其机制可能与厚壁菌门/拟杆菌门比值升高有关,两者均可促进疾病进展。这些因素之间的因果关系以及这种关联背后的上下游机制仍然难以捉摸,需要进一步研究。值得注意的是,一些调查在临床和临床前环境中得出了对比鲜明但似乎合理的结果 [9]。Zhang等 [14]进行了16S rRNA基因测序,并阐明了对照组中Blautia和厌氧菌的富集,这是两种影响体内平衡和抗炎症的重要短链脂肪酸产生细菌。许多厚壁菌门对年龄相关性黄斑变性和其他疾病有益。门是一个广泛的细菌分类,包括各种细菌;因此,一个门究竟是完全好的还是完全坏的,需要研究。此外,探索每种细菌的特定功能对于更好地阐明厚壁菌门/拟杆菌门在年龄相关性黄斑变性中的功能至关重要。例如,多项研究表明,在年龄相关性黄斑变性患者中,拟杆菌门和变形杆菌门的促炎细菌明显增加,而产丁酸细菌明显减少。
此外,这些研究也存在一定的局限性。首先,有限的样本量和混杂因素严重影响了结果的准确性和可重复性,导致研究存在异质性。其次,不同研究中样品种类和检测方法的差异阻碍了对肠道菌群功能的直接比较。随着高通量测序技术的进步,更精确的检测技术可能会出现,扩大肠道菌群研究的范围,提高研究结果的可比性。
综述的重点已从研究肠道菌群组成的变化转向研究特定肠道菌群及其代谢物对宿主的影响。肠道菌群是人体的内分泌器官,其代谢产物可与人体相互作用。许多代谢物可以到达并影响视网膜。一些代谢组学研究也对年龄相关性黄斑变性进行了研究,强调代谢物对年龄相关性黄斑变性发病机制的影响 [15, 16]。这些代谢物驱动机体与体内菌群之间的稳态,影响疾病的发生和进展(图1)。脂多糖是革兰氏阴性菌细胞壁中的一种特有成分,是一种重要的与病原体相关的分子模式分子。当细菌与人类或动物细胞相互作用时,它们会从表面释放脂多糖。脂多糖与TLR4形成蛋白复合物,将促炎信号传递到细胞中。随后,该复合物激活关键的信号级联,包括核因子kappa-B和丝裂原活化蛋白激酶,触发促炎介质的合成。这些因素引发慢性炎症反应,增加氧化应激。年龄相关性黄斑变性患者中某些产生脂多糖的促炎细菌升高,包括埃希氏志贺氏菌和细微杆菌。同样,有研究表明年龄相关性黄斑变性患者肠道菌群的变化与脂多糖合成代谢密切相关。且脂多糖参与小胶质细胞介导的神经炎症,因此年龄相关性黄斑变性患者肠道菌群失调可能通过触发脂多糖-TLR4通路和随后的小胶质细胞介导的神经炎症来促进疾病的进展。色氨酸是一种人体不可内源性合成的必需氨基酸,是富含蛋白质的食物的基本组成部分。大多数膳食色氨酸是通过犬尿氨酸途径代谢的,其下游代谢物在神经退行性疾病中至关重要。乳杆菌和梭状芽孢杆菌可以从色氨酸生产吲哚衍生物,如吲哚-3-乳酸和吲哚-3-丙酸,从而激活芳烃受体,并通过多种途径影响年龄相关性黄斑变性的进展,包括小胶质细胞激活、视网膜组织稳态和脉络膜新生血管的形成。在肠道菌群中,甜菜碱、肉碱和胆碱等化合物通过三甲胺裂解酶进行酶解。随后,这些代谢物在肝脏中被称为含黄素二甲基苯胺单加氧酶的酶家族氧化,导致氧化三甲胺的形成。三甲胺-氧化三甲胺通路是饮食、肠道菌群和疾病之间的新交叉。研究表明,厚壁菌门和变形菌门的不同属可以影响三甲胺和氧化三甲胺的产生。例如,芽孢杆菌、志贺氏菌和振荡菌与氧化三甲胺代谢呈正相关,而乳酸杆菌和双歧杆菌与血清氧化三甲胺水平呈负相关 [17]。据报道,阿尔茨海默病患者的血浆和脑脊液中氧化三甲胺水平较高。此外,氧化三甲胺水平与阿尔茨海默病病理中神经元变性的几种生物标志物有关。因此,肠道菌群的改变可能导致氧化三甲胺代谢紊乱,加重年龄相关性黄斑变性。氧化三甲胺代谢紊乱可能以多种方式促进年龄相关性黄斑变性的进展,如影响脂质代谢,激活炎症信号通路,影响血视网膜屏障完整性。优化肠道菌群结构是针对氧化三甲胺的一种可行有效的方法。短链脂肪酸作为肠道菌群中最常见的代谢物,可以通过激活G蛋白偶联受体控制炎症和调节代谢,使多种细胞受益。短链脂肪酸可以在维持屏障的完整性、调节小胶质细胞稳态、阻碍淀粉样蛋白-肽聚集等方面发挥作用。此外,短链脂肪酸可以抑制组蛋白去乙酰化酶,调节基因表达和生理过程。大量关于年龄相关性黄斑变性患者肠道菌群的研究发现,在年龄相关性黄斑变性患者的肠道菌群中,产生短链脂肪酸的细菌(如Blautia)发生了改变,这些细菌在年龄相关性黄斑变性患者的肠道菌群中显著下调。因此,短链脂肪酸水平的变化可能通过多种途径影响年龄相关性黄斑变性的发病机制,包括drusen形成、血视网膜屏障完整性和免疫稳态,影响疾病的发生和进展。因此,针对短链脂肪酸的治疗策略有望有效治疗年龄相关性黄斑变性。胆汁酸是胆汁的主要有机成分。肠道菌群与胆汁酸之间复杂的相互作用形成了一个动态平衡,显著影响胆汁酸代谢和复杂的肠肝循环轴。此外,胆汁酸及其受体可以影响肠道菌群的组成和密度,不同程度地抑制有害细菌的过度生长和迁移。胆汁酸通过与各种受体结合来调节新陈代谢和免疫。例如,通过激活法内酯X受体,胆汁酸调节脂质代谢和脑胆固醇积累。同时,牛磺脱氧胆酸和熊去氧胆酸可能通过减轻线粒体功能障碍、防止细胞凋亡、保护视网膜色素上皮和感光细胞参与年龄相关性黄斑变性的发病机制。这些代谢物对年龄相关性黄斑变性的影响,其具体机制大多与免疫炎症反应、胶质细胞活化、补体级联等密不可分。胶质细胞活化是年龄相关性黄斑变性发病的重要因素。此外,在许多遗传和分子研究中,补体系统被认为是年龄相关性黄斑变性的一个重要因素 [18]。尽管取得了重大进展,但我们对这些肠道衍生代谢物的理解仍处于初级阶段,其中大部分仍未被识别,其多种功能在很大程度上难以捉摸。复杂的潜在机制需要深入探索。此外,肠道菌群与其代谢物之间并没有一对一的关系。许多代谢物不是肠道菌群所独有的,它们的具体功能尚不清楚。因此,进一步研究肠道菌群及其代谢产物在年龄相关性黄斑变性中的具体作用,并阐明其可能的机制是必要的。
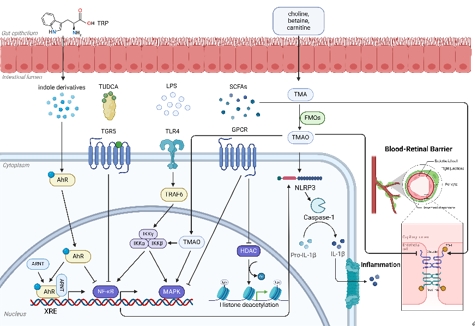
图1 肠道菌群代谢产物对人体的影响(图源:Jin et al., Neural Regen Res, 2025)
早期年龄相关性黄斑变性缺乏可靠和有效的治疗方案。因此,在早期阶段预防其发展是至关重要的。鉴于肠道菌群在年龄相关性黄斑变性发病机制中的重要性,考虑针对这方面的治疗和预防策略是至关重要的。然而,由于研究数据有限,每种治疗方法的安全性和有效性需要临床评估。研究发现,西方高糖高脂的饮食模式可能导致年龄相关性黄斑变性进展,而富含维生素和抗氧化剂的地中海饮食模式可以很好地抑制年龄相关性黄斑变性。此外,间断性禁食可以改变肠道菌群的组成,丰富益生菌,从而减轻体重,降低血压,并有助于预防癌症,心血管疾病和代谢疾病,抑制年龄相关性黄斑变性的进展。而直接针对有益和有害菌群采取相应措施在神经退行性疾病中也得到了很多应用,而在年龄相关性黄斑变性中的作用仍需进一步探索。其中,粪菌移植是近些年新兴的一种靶向肠道菌群的方法。考虑到其复杂的操作程序和昂贵的成本,需要对其短期和长期的安全性和有效性进行评估。针对肠道菌群的多种治疗可以影响神经退行性疾病,有效促进神经再生等过程。因此,我们假设年龄相关性黄斑变性可以通过简单的方法来得到抑制,如改变饮食,使用益生菌和抗生素,以及粪便微生物移植。
许多研究发现年龄相关性黄斑变性患者的厚壁菌门/拟杆菌门比值有所增加。在特定细菌方面,年龄相关性黄斑变性患者的产短链脂肪酸的细菌显著减少,而拟杆菌门和变形杆菌门中的许多促炎细菌显著增加。肠道菌群的改变涉及的机制包括脂质异常、氧化应激和小胶质细胞与其他细胞之间的串扰。后续的研究工作应该集中在阐明不同细菌的具体贡献,从而揭示肠道菌群与年龄相关性黄斑变性之间直接或间接关联的复杂机制。在治疗肠道菌群的各种方法中,最简单可行的是饮食策略,益生菌,抗生素和FMT是有前途的治疗。需要进一步的研究来确定这些新方法的可行性和有效性,从而为年龄相关性黄斑变性的早期干预选择最合适的方法、有效的治疗窗期和最佳的治疗剂量。同样,个体微生物组是独一无二的,个体肠道微生物组的针对性改善对于推进个性化医疗至关重要,特别是在治疗神经退行性疾病方面。
目前,关于肠道菌群在年龄相关性黄斑变性中的作用的直接研究仍然相对较少,而对肠道菌群的单独研究不足以充分阐明其在年龄相关性黄斑变性中的功能作用。器官芯片技术在阐明肠道-眼相互作用方面有希望,通过代谢组学和其他技术将肠道菌群与代谢物结合起来对于阐明其潜在机制至关重要。我们正在等待针对肠道菌群的靶向治疗干预的发展,以安全有效地阻止年龄相关性黄斑变性的早期进展。
原文链接:https://doi.org/10.4103/NRR.NRR-D-24-00531
参考文献
[1] Cryan JF, O'riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194.
[2] Wijesekara T, Abeyrathne E, Ahn DU. Effect of bioactive peptides on gut microbiota and their relations to human health. Foods. 2024;13(12):1853.
[3] Papadopoulos Z. Neovascular age-related macular degeneration and its association with Alzheimer's disease. Curr Aging Sci. 2020;13(2):102-112.
[4] Zinkernagel MS, Zysset-Burri DC, Keller I, et al. Association of the intestinal microbiome with the development of neovascular age-related macular degeneration. Sci Rep. 2017;7:40826.
[5] Zysset-Burri DC, Keller I, Berger LE, et al. Associations of the intestinal microbiome with the complement system in neovascular age-related macular degeneration. NPJ Genom Med. 2020;5:34.
[6] Li C, Lu P. Association of gut microbiota with age-related macular degeneration and glaucoma: a bidirectional mendelian randomization study. Nutrients. 2023;15(21):4646.
[7] Mao D, Tao B, Sheng S, et al. Causal effects of gut microbiota on age-related macular degeneration: a mendelian randomization study. Invest Ophthalmol Vis Sci. 2023;64(12):32.
[8] Andriessen EM, Wilson AM, Mawambo G, et al. Gut microbiota influences pathological angiogenesis in obesity-driven choroidal neovascularization. EMBO Mol Med. 2016;8(12):1366-1379.
[9] Cao Y, Ibrahim KS, Li X, et al. Chinese medicine, Qijudihuang pill, mediates cholesterol metabolism and regulates gut microbiota in high-fat diet-fed mice, implications for age-related macular degeneration. Front Immunol. 2023;14:1274401.
[10] Li Y, Cai Y, Huang Q, et al. Altered fecal microbiome and metabolome in a mouse model of choroidal neovascularization. Front Microbiol. 2021;12:738796.
[11] Rowan S, Jiang S, Korem T, et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci U S A. 2017;114(22):E4472-E4481.
[12] Kiang L, Mcclintic S, Saleh M, et al. The gut microbiome in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(8):5739-5739.
[13] Xue W, Peng P, Wen X, et al. Metagenomic sequencing analysis identifies cross-cohort gut microbial signatures associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64(5):11.
[14] Zhang Y, Wang T, Wan Z, et al. Alterations of the intestinal microbiota in age-related macular degeneration. Front Microbiol. 2023;14:1069325.
[15] Acar İ E, Lores-Motta L, Colijn JM, et al. Integrating metabolomics, genomics, and disease pathways in age-related macular degeneration: The EYE-RISK Consortium. Ophthalmology. 2020;127(12):1693-1709.
[16] Han X, Lains I, Li J, et al. Integrating genetics and metabolomics from multi-ethnic and multi-fluid data reveals putative mechanisms for age-related macular degeneration. Cell Rep Med. 2023;4(7):101085.
[17] Yi ZY, Peng YJ, Hui BP, et al. Zuogui-Jiangtang-Yishen decoction prevents diabetic kidney disease: Intervene pyroptosis induced by trimethylamine n-oxide through the mROS-NLRP3 axis. Phytomedicine. 2023;114:154775.
[18] Armento A, Ueffing M, Clark SJ. The complement system in age-related macular degeneration. Cell Mol Life Sci. 2021;78(10):4487-4505.



