NRR:中国海军军医大学长海医院杨鹏飞和海军特色医学中心张萍团队总结脂质代谢及小胶质细胞参与脑卒中预防和治疗的新策略
撰文:陈蕾
#br#
小胶质细胞和脂质代谢在脑卒中的发病机制和预后过程中都起着重要作用。脑卒中损伤后的小胶质细胞对环境变化具有敏感的感知能力,激活的小胶质细胞经历表型重塑并触发复杂的信号级联来调节脑卒中后的免疫反应 [1]。小胶质细胞对脑卒中后的免疫调节作用影响了脑卒中的结局。脂质代谢被认为是脑卒中发病过程中的重要影响因素,也是脑卒中预防和治疗的关键靶点。脂质代谢异常与脑动脉粥样硬化的相关性已经被广泛认识到 [2]。但近年来的研究发现,脂质代谢不仅影响了血管斑块形成,脂质包括外周脂质代谢和脂滴生物生成,还参与控制小胶质细胞的功能,如活化、吞噬、增殖和炎症反应 [3]。饮食和肠道微生物群通过肠脑轴也对神经炎症系统起到非常重要的影响作用。这些脑卒中相关的脂质代谢和小胶质细胞调节炎症的各个环节,为脑保护治疗提供了新的治疗靶点。但目前对小胶质细胞和脂质代谢在脑卒中预防和治疗中的作用机制和治疗靶点缺乏一个完整的全面的总结。
中国海军军医大学长海医院杨鹏飞和海军特色医学中心张萍团队在《中国神经再生研究(英文)》(Neural Regeneration
Research)上发表了题为“Lipid
metabolism, microglia, and stroke”的综述。文章总结了脂质代谢对脑卒中的免疫调节作用,并讨论了小胶质细胞与脂质的相互作用,特别关注了饮食和肠道微生物如何通过肠脑轴影响神经炎症,分析脑卒中后脂质代谢和小胶质细胞调节炎症相关的治疗靶点及临床研究进展。这为更有效和更安全的脑卒中的预防和脑保护治疗提供前瞻性策略。
脑卒中的病理过程,包括缺血和出血,涉及一系列反应,如小胶质细胞激活、炎症、氧化应激和外周免疫细胞募集,在数小时到数天甚至数周内不断演变 [4, 5]。急性血管性脑损伤后发生复杂免疫级联反应,产生细胞因子、趋化因子和其他调节分子,促进或减轻脑损伤,并参与随后的脑功能恢复。脑卒中后,小胶质细胞在神经元细胞死亡前迅速激活,在先天免疫中发挥重要作用 [1, 6]。然后,进展中的神经炎症对大脑造成额外的损伤,导致细胞在急性期死亡,但反过来在修复活动中起有益作用,并促进脑卒中后修复阶段的恢复。小胶质细胞和脂质代谢介导神经营养因子的分泌和碎片的清除促进了脑组织的修复 [7, 8]。脂质是脑组织的主要成分,对维持中枢神经系统的正常功能和稳态至关重要。胆固醇、磷脂、糖脂和脂肪酸等各种脂类也是细胞膜的主要成分 [9]。脑缺血时由于脑血流严重不足,稳态必需脂质代谢受损 [10, 11],产生各种脂质介质参与缺血性损伤的促炎和促细胞溶解过程的调节 [12]。大量证据表明,高水平的血浆胆固醇与更大的梗死面积和更高程度的水肿有关 [13-16]。高脂血症与脑卒中后病灶内促炎反应的增加密切相关 [17]。
脂质在生理功能中的作用是非常重要的。胆固醇不仅有助于细胞膜的稳定,而且还有助于细胞膜之间的运输。大脑具有高浓度的脂质,特别是多不饱和脂肪酸。多不饱和脂肪酸是大脑发育、记忆能力和学习的重要生物活性营养素 [18]。脂质也是维持脑细胞结构完整性、影响细胞膜流动性和通透性、促进能量代谢和支持信号通路的关键 [19]。脂代谢异常被认为脑卒中的重要危险因素之一。脂质代谢紊乱在脑动脉粥样硬化血管斑块形成中起着关键性的作用。近年来,脂质代谢在脑卒中的发展和脑功能恢复中被发现有新的关键性的作用机制。
最近有研究表明,脂质代谢在调节小胶质细胞功能,包括小胶质细胞激活、增殖、吞噬和炎症信号转导中可能起作用 [20, 21]。脑卒中诱导的小胶质细胞激活与脂滴聚集有关 [22, 23]。脂滴是储存脂肪酸并具有多种调节功能的小细胞器 [24]。缺血第3天,小胶质细胞聚集在局灶性缺血性病变周围,产生的炎症引起有利于脂质合成的代谢变化 [23]。小胶质细胞的脂质合成代谢和吞噬性脂质摄入驱动脂滴生物发生,增加炎症,并使小胶质细胞增殖 [25, 26]。增殖的小胶质细胞分泌营养因子,有助于缺血组织的保护和修复。然而,一些持续积累脂滴的小胶质细胞会转化为功能失调和有潜在危害的泡沫细胞 [27]。有报道称,小胶质细胞内脂滴的积累与其吞噬作用呈负相关。抑制小胶质细胞中脂滴的形成可恢复其在阿尔茨海默病中的吞噬功能 [28]。缺血性脑卒中后,许多因素可引起小胶质细胞的脂滴合成,包括炎症环境和细胞碎片的吞噬(图1)。
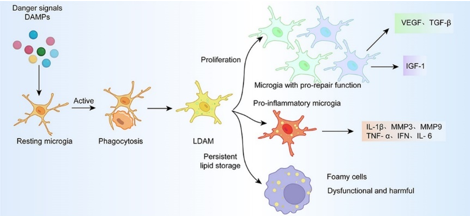
图1脂滴对脑卒中后小胶质细胞的功能调控
以脂质代谢的重大变化为特征的代谢紊乱和肥胖等疾病中,小胶质细胞炎症的激活表现突出 [29, 30]。多项研究证明,高脂肪饮食改变了小胶质细胞依赖性脑炎症环境 [30-33],但与体质量改变无关 [34, 35]。脑卒中模型中,长时间高脂肪饮食诱导的肥胖动物模型脑内小胶质细胞/巨噬细胞增加。高脂肪饮食模型中炎症反应的改变可能导致脑卒中后更严重的神经损伤 [36]。高脂肪饮食诱导的肥胖通过影响梗死区域内神经元、小胶质细胞/巨噬细胞和星形胶质细胞的分布而加剧脑卒中损害 [37]。脑缺血后产生的多不饱和脂肪酸及其代谢物等多种脂质介质可以改变炎症过程,促进脑缺血后的回归过程 [38]。小胶质细胞在不同疾病和阶段的吞噬反应也可能受到多不饱和脂肪酸的差异调节 [7]。适度的饮食蛋白质限制可以防止缺血性脑的炎症和氧化反应损伤 [39]。脑缺血后,脑损伤导致自主神经系统来源的自主去甲肾上腺素释放,减少肠上皮细胞数量,破坏粘液分泌功能,使肠道微生物群落进入生态失调状态 [40]。而肠道微生物群可以通过肠脑轴调节小胶质细胞功能 [41]。
积极的脑保护治疗对脑卒中的恢复至关重要。神经炎症一直是重要的治疗靶点。脂质代谢紊乱和脑卒中后神经炎症显著影响脑卒中恢复。小胶质细胞对神经炎症至关重要,与脂质通路有重要的相互作用,因此靶向脂质代谢和小胶质细胞功能是一种很有前景的治疗策略。目前已经提出了几种新的治疗方法,包括药物和营养干预。通过降低炎症标志物如C反应蛋白,他汀类药物可能有助于降低脑卒中风险,增强内皮功能,减少氧化应激,并提供抗炎作用 [42]。PCSK9抑制剂可减轻动脉粥样硬化血管内的炎症负担 [43, 44],逆转高胆固醇血症患者的促炎反应,减少低密度脂蛋白胆固醇引起的全身炎症,并减少脑血管事件 [45]。
在脑卒中各时点控制小胶质细胞极化可以通过促进M2表型和阻断M1表型来减少神经炎症,从而精准调控脑卒中后抗炎和促炎反应的平衡。PPAR激动剂可减少氧化应激和炎症反应,改善脑卒中动物模型的神经预后 [45]。姜黄素已被证明可以影响M1和M2表型之间的平衡,为缺血性脑卒中提供保护 [46]。α-硫辛酸已被证明可以影响小胶质细胞的M1/M2极化,降低促炎细胞因子表达,提高抗炎细胞因子水平,阻断NF-κB信号通路,从而具有神经保护作用 [47]。在小鼠脑出血模型中,紫檀芪显示出减轻神经炎症和脑损伤的潜力 [48]。人脂肪源性间充质干细胞外泌体可显著增强神经功能,减少神经元凋亡,并影响小胶质细胞极化 [49]。脂质纳米颗粒有望作为基于脂质的靶向药物递送系统,用于调节小胶质细胞活性 [49]。
从目前已经开展临床应用研究的药物前景来看,除了已经应用于临床的他汀和PCSK9抑制剂,多不饱和脂肪酸助于调节脂质代谢和炎症反应,减少动脉粥样硬化和神经炎症,从而降低脑卒中风险 [50],美国心脏协会和美国脑卒中协会推荐高剂量的ω-3多不饱和脂肪酸用于预防心脏病患者复发,这对脑卒中患者也可能有相应的益处 [51]。此外,一项荟萃分析表明,饮食中摄入更多的维生素E与降低脑卒中风险有关 [52]。维生素E和硒等抗氧化剂在减轻氧化应激方面具有重要作用,而氧化应激与各种健康问题有关,包括脂质失调和神经胶质细胞功能障碍。在加拿大的因纽特人中,较高的饮食和血液硒水平与较低的脑卒中患病率有关 [53]。维生素E和硒作为膳食抗氧化剂,可能与积极有益的健康结果有关 [54]。但目前支持脑卒中后常规临床应用的证据不足 [52, 55]。
脑卒中后脂质代谢和小胶质细胞调控治疗仍然面临着许多挑战和问题。脑卒中后病理的动态性改变使干预的时机复杂化。小胶质细胞在脑卒中后迅速激活,在不同阶段表现出不同的表型。针对脂质代谢和小胶质细胞调节的干预时机至关重要。治疗效果可能因时间不同而有显著差异,因此需要进一步研究以确定最佳治疗时机。患者个体化差异也是影响脑卒中治疗结果的主要因素。年龄、性别、遗传背景和合并症(如糖尿病、高血压和高脂血症)可能通过影响脂质代谢和小胶质细胞功能来改变治疗效果 [56]。血脑屏障仍然是向大脑输送药物或基因治疗的一个重要障碍。技术传输的创新方法包括利用三叉神经和嗅觉途径的鼻内输送,以及通过聚焦超声与微泡结合临时打开血脑屏障的技术 [57-59]。细胞穿透肽可以通过促进药物在内皮细胞间的转运来增强药物的吸收 [60],但可扩展性和安全性需要进一步验证。以脂质代谢或小胶质细胞活性为靶点还有全身副作用的风险,包括免疫抑制或代谢紊乱。调节小胶质细胞的活性可以影响更广泛的免疫系统,可能导致对感染或自身免疫反应的易感性增加 [61],也可能引发全身性炎症,或者相反,抑制必要的炎症反应,导致伤口愈合不良或慢性炎症 [62]。平衡疗效与安全性对临床转化至关重要。由于小胶质细胞对大脑稳态至关重要,靶向它们的活动还可能会导致意想不到的神经系统副作用,包括认知障碍、情绪障碍或神经退行性疾病的加剧 [63, 64]。调节脂质代谢或小胶质细胞活性的长期影响尚不完全清楚,并且可能在干预后数年出现延迟的不良反应。
目前对脑卒中炎症和免疫反应的机制还远远没有深入了解。小胶质细胞和脂质代谢在脑卒中后炎症中的作用是一把双刃剑,它既是脑卒中后脑损伤的关键因素,又可以抑制炎症修复受损的大脑。越来越多的证据表明,神经炎症中的小胶质细胞和脂质在缺血性脑卒中的发病机制中起着关键作用,并已成为治疗干预的潜在靶点。但目前很多研究结果仍然停留于细胞水平和动物模型阶段,需要更多的临床研究和真实世界结果来验证这些干预手段的效果和副作用。将来我们需要更好地了解炎症和免疫系统在脑卒中中的作用,利用基因组学和脂质组学等新技术,以达到调节炎症系统和创新治疗策略的最终目的。未来药物研发会有更多方向。将脂质靶向药物与抗炎药物联合使用可能会产生协同效应;改善治疗效果而不会产生过多的副作用;组学发现和成像技术的进步可能使针对脂质代谢和小胶质细胞功能的精准医学成为可能;人工智能可以加速识别新的候选药物,优化脑卒中治疗的递送策略。通过解决这些局限性并利用尖端技术,调节脂质代谢和小胶质细胞作为脑卒中的治疗策略具有巨大的潜力。
原文链接:https://doi.org/10.4103/NRR.NRR-D-24-01523
#br#
参考文献
[1] Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest. 2020;130(6):2777-2788.
[2] Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn). 2017;23(1, Cerebrovascular Disease):15-39.
[3] Planas AM. Role of microglia in stroke. Glia. 2024;72(6):1016-1053.
[4] Kim SJ, Nogueira RG, Haussen DC. Current understanding and gaps in research of carotid webs in ischemic strokes: a review. JAMA Neurol. 2019;76(3):355-361.
[5] Bai Q, Xue M, Yong VW. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: therapeutic opportunities. Brain. 2020;143(5):1297-1314.
[6] Li Y, Wang Y, Yao Y, et al. Systematic study of the immune components after ischemic stroke using CyTOF techniques. J Immunol Res. 2020;2020:9132410.
[7] Leyrolle Q, Layé S, Nadjar A. Direct and indirect effects of lipids on microglia function. Neurosci Lett. 2019;708:134348.
[8] Delong JH, Ohashi SN, O'connor KC, et al. Inflammatory responses after ischemic stroke. Semin Immunopathol. 2022;44(5):625-648.
[9] Andreone BJ, Chow BW, Tata A, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94(3):581-594.e5.
[10] Paik MJ, Li WY, Ahn YH, et al. The free fatty acid metabolome in cerebral ischemia following human mesenchymal stem cell transplantation in rats. Clin Chim Acta. 2009;402(1-2):25-30.
[11] Shanta SR, Choi CS, Lee JH, et al. Global changes in phospholipids identified by MALDI MS in rats with focal cerebral ischemia. J Lipid Res. 2012;53(9):1823-1831.
[12] Nakamura A, Otani K, Shichita T. Lipid mediators and sterile inflammation in ischemic stroke. Int Immunol. 2020;32(11):719-725.
[13] Kim E, Tolhurst AT, Qin LY, et al. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28(18):4661-4670.
[14] Navi BB, Segal AZ. The role of cholesterol and statins in stroke. Curr Cardiol Rep. 2009;11(1):4-11.
[15] Elali A, Doeppner TR, Zechariah A, et al. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke. 2011;42(11):3238-3244.
[16] Kim E, Yang J, Woo Park K, et al. Preventative, but not post-stroke, inhibition of CD36 attenuates brain swelling in hyperlipidemic stroke. J Cereb Blood Flow Metab. 2020;40(4):885-894.
[17] Kim E, Febbraio M, Bao Y, et al. CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol. 2012;71(6):753-764.
[18] Hamed M, Martyniuk CJ, Naguib M, et al. Neurotoxic effects of different sizes of plastics (nano, micro, and macro) on juvenile common carp (Cyprinus carpio). Front Mol Neurosci. 2022;15:1028364.
[19] Hoscheidt S, Sanderlin AH, Baker LD, et al. Mediterranean and Western diet effects on Alzheimer's disease biomarkers, cerebral perfusion, and cognition in mid-life: a randomized trial. Alzheimers Dement. 2022;18(3):457-468.
[20] Krasemann S, Madore C, Cialic R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47(3):566-581.e569.
[21] Chausse B, Kakimoto PA, Kann O. Microglia and lipids: how metabolism controls brain innate immunity. Semin Cell Dev Biol. 2021;112:137-144.
[22] Lin CH, Liao LY, Yang TY, et al. Microglia-derived adiposomes are potential targets for the treatment of ischemic stroke. Cell Mol Neurobiol. 2019;39(5):591-604.
[23] Arbaizar-Rovirosa M, Pedragosa J, Lozano JJ, et al. Aged lipid-laden microglia display impaired responses to stroke. EMBO Mol Med. 2023;15(2):e17175.
[24] Bosch M, Parton RG, Pol A. Lipid droplets, bioenergetic fluxes, and metabolic flexibility. Semin Cell Dev Biol. 2020;108:33-46.
[25] Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033.
[26] O'neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553-565.
[27] Beccari S, Sierra-Torre V, Valero J, et al. Microglial phagocytosis dysfunction in stroke is driven by energy depletion and induction of autophagy. Autophagy. 2023;19(7):1952-1981.
[28] Wu X, Miller JA, Lee BTK, et al. Reducing microglial lipid load enhances β amyloid phagocytosis in an Alzheimer's disease mouse model. Sci Adv. 2025;11(6):eadq6038.
[29] Gao Y, Vidal-Itriago A, Kalsbeek MJ, et al. Lipoprotein lipase maintains microglial innate immunity in obesity. Cell Rep. 2017;20(13):3034-3042.
[30] Cope EC, Lamarca EA, Monari PK, et al. Microglia play an active role in obesity-associated cognitive decline. J Neurosci. 2018;38(41):8889-8904.
[31] Maldonado-Ruiz R, Montalvo-Martínez L, Fuentes-Mera L, et al. Microglia activation due to obesity programs metabolic failure leading to type two diabetes. Nutr Diabetes. 2017;7(3):e254.
[32] Valdearcos M, Douglass JD, Robblee MM, et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26(1):185-197.e3.
[33] Ullah R, Rauf N, Nabi G, et al. Mechanistic insight into high-fat diet-induced metabolic inflammation in the arcuate nucleus of the hypothalamus. Biomed Pharmacother. 2021;142:112012.
[34] Sharma S. High fat diet and its effects on cognitive health: alterations of neuronal and vascular components of brain. Physiol Behav. 2021;240:113528.
[35] Wang XL, Li L. Microglia regulate neuronal circuits in homeostatic and high-fat diet-induced inflammatory conditions. Front Cell Neurosci. 2021;15:722028.
[36] Maysami S, Haley MJ, Gorenkova N, et al. Prolonged diet-induced obesity in mice modifies the inflammatory response and leads to worse outcome after stroke. J Neuroinflammation. 2015;12:140.
[37] Fifield KE, Rowe TM, Raman-Nair JB, et al. Prolonged high fat diet worsens the cellular response to a small, covert-like ischemic stroke. Neuroscience. 2019;406:637-652.
[38] Reid MM, Belayev L, Khoutorova L, et al. Integrated inflammatory signaling landscape response after delivering Elovanoid free-fatty-acid precursors leading to experimental stroke neuroprotection. Sci Rep. 2023;13(1):15841.
[39] De Carvalho TS, Sanchez-Mendoza EH, Nascentes LM, et al. Moderate protein restriction protects against focal cerebral ischemia in mice by mechanisms involving anti-inflammatory and anti-oxidant responses. Mol Neurobiol. 2019;56(12):8477-8488.
[40] Tan C, Wu Q, Wang H, et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. JPEN J Parenter Enteral Nutr. 2021;45(3):518-529.
[41] Arya AK, Hu B. Brain-gut axis after stroke. Brain Circ. 2018;4(4):165-173.
[42] Choudhary A, Rawat U, Kumar P, et al. Pleotropic effects of statins: the dilemma of wider utilization of statin. Egypt Heart J. 2023;75(1):1.
[43] Barale C, Melchionda E, Morotti A, et al. PCSK9 biology and its role in atherothrombosis. Int J Mol Sci. 2021;22(11):5880.
[44] Marfella R, Prattichizzo F, Sardu C, et al. Evidence of an anti-inflammatory effect of PCSK9 inhibitors within the human atherosclerotic plaque. Atherosclerosis. 2023;378:117180.
[45] Marques P, Domingo E, Rubio A, et al. Beneficial effects of PCSK9 inhibition with alirocumab in familial hypercholesterolemia involve modulation of new immune players. Biomed Pharmacother. 2022;145:112460.
[46] Liu Z, Ran Y, Huang S, et al. Curcumin protects against ischemic stroke by titrating microglia/macrophage polarization. Front Aging Neurosci. 2017;9:233.
[47] Wang Q, Lv C, Sun Y, et al. The role of alpha-lipoic acid in the pathomechanism of acute ischemic stroke. Cell Physiol Biochem. 2018;48(1):42-53.
[48] Wu Y, Hu Q, Wang X, et al. Pterostilbene attenuates microglial inflammation and brain injury after intracerebral hemorrhage in an OPA1-dependent manner. Front Immunol. 2023;14:1172334.
[49] Zhao L, Li J. Microglial uptake of hADSCs-Exo mitigates neuroinflammation in ICH. Cell Signal. 2024;119:111146.
[50] Bae JH, Lim H, Lim S. The potential cardiometabolic effects of long-chain ω-3 polyunsaturated fatty acids: recent updates and controversies. Adv Nutr. 2023;14(4):612-628.
[51] Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364-e467.
[52] Cheng P, Wang L, Ning S, et al. Vitamin E intake and risk of stroke: a meta-analysis. Br J Nutr. 2018;120(10):1181-1188.
[53] Hu XF, Sharin T, Chan HM. Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada. J Trace Elem Med Biol. 2017;44:322-330.
[54] Myhrstad MCW, Wolk A. Antioxidants and phytochemicals - a scoping review for Nordic Nutrition Recommendations 2023. Food Nutr Res. 2023;67:10324.
[55] Hantikainen E, Lagerros YT. Vitamin E - a scoping review for Nordic Nutrition Recommendations 2023. Food Nutr Res. 2023;67:10238.
[56] Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179(2):292-311.
[57] Kulkarni M, Patel K, Patel A, et al. Nanomaterials as drug delivery agents for overcoming the blood-brain barrier: A comprehensive review. ADMET DMPK. 2024;12(1):63-105.
[58] López-Aguirre M, Castillo-Ortiz M, Viña-González A, et al. The road ahead to successful BBB opening and drug-delivery with focused ultrasound. J Control Release. 2024;372:901-913.
[59] Peng B, Mohammed FS, Tang X, et al. Nanotechnology approaches to drug delivery for the treatment of ischemic stroke. Bioact Mater. 2025;43:145-161.
[60] Ayuso-Dolado S, Esteban-Ortega GM, Vidaurre Ó G, et al. A novel cell-penetrating peptide targeting calpain-cleavage of PSD-95 induced by excitotoxicity improves neurological outcome after stroke. Theranostics. 2021;11(14):6746-6765.
[61] Zhang W, Xu H, Li C, et al. Exploring Chinese herbal medicine for ischemic stroke: insights into microglia and signaling pathways. Front Pharmacol. 2024;15:1333006.
[62] Zhang G, Xia F, Zhang Y, et al. Ginsenoside Rd is efficacious against acute ischemic stroke by suppressing microglial proteasome-mediated inflammation. Mol Neurobiol. 2016;53(4):2529-2540.
[63] Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018-1027.
[64] Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619-643.
