中国神经再生研究(英文版) ›› 2026, Vol. 21 ›› Issue (4): 1641-1651.doi: 10.4103/NRR.NRR-D-25-00037
Cullin3-Ring E3泛素连接酶与USP14介导Spastin泛素/去泛素化修饰调控微管切割和神经元生长的新机制
The Cullin3–Ring E3 ubiquitin ligase complex and USP14 regulate spastin-mediated microtubule severing and promotion of neurite outgrowth
Zhenbin Cai1, #, Hui Wu1, #, Tao Jiang2, #, Ao Ma1 , Zhichao Meng1 , Jiehao Zhu1 , Hongsheng Lin1 , Yaozhong Liang1, *, Guowei Zhang1, *, Minghui Tan1, *
- 1 Department of Orthopedics, The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong Province, China; 2 Department of Orthopedics, Guangzhou Eighth People’s Hospital of Guangzhou Medical University, Guangzhou, Guangdong Province, China
摘要:
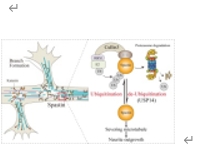
微管切割蛋白Spastin的后修饰导致了对微管切断活性的精确时空控制。然而,在神经元生长过程中,Spastin 蛋白更替的详细机制仍不清楚。实验发现spastin与泛素相互作用,并通过K48介导的多泛素化显著降解。Cullin3促进了Spastin的降解和泛素化。RBX1(而非RBX2)与Cullin3蛋白共同调控Spastin的降解。过量表达Culin3或BRX1都会显著抑制spastin的蛋白水平,并抑制Spastin介导的微管切断和促进神经元生长的作用。此外,还发现 USP14 介导了 spastin 的去泛素化。USP14 与 Spastin 相互作用。过量表达 USP14 能显著提高蛋白水平,抑制 Spastin 的泛素化和降解。虽然Spastin和USP14的共同表达并不能增强微管的切断,但却能增加海马神经元的神经元长度。总之,实验结果阐明了Spastin周转的复杂调控机制,强调了Cullin-3-Ring E3泛素连接酶复合物和USP14在协调其降解和泛素化调控中的作用。这些因素之间的动态相互作用决定了海绵蛋白的稳定性和功能性,并最终影响微管动力学和神经元形态。这些发现为Spastin相关神经退行性疾病的治疗提供了新的分子机制和潜在干预策略。
https://orcid.org/000-0002-1655-6498 (Guowei Zhang); https://orcid.org/0009-0003-1884-7648 (Yaozhong Liang); https://orcid.org/0000-0001-6260-4115 (Minghui Tan)