视神经损伤
-
Figure 1|TSPO is localized to RPE cells.
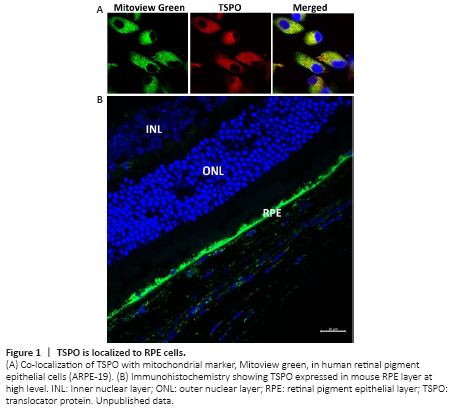
Removal of additional cholesterol from retina and RPE is mediated by reverse cholesterol transport which helps to return cellular cholesterol to the liver for either storage or excretion. The translocator protein (TSPO) localizes to mitochondrial outer membrane with five transmembrane domains. TSPO can bind cholesterol via the cholesterol recognition amino acid consensus on the fifth transmembrane domain and is mainly responsible for transferring cholesterol from the mitochondrial outer membrane to the mitochondrial inner membrane, where cholesterol is converted into pregnenolone in steroidogenic cells and into oxysterols in non-steroidogenic cells such as the RPE and macrophages. Oxysterols can activate the LXRα signaling pathway, upregulating cholesterol transporting genes and increasing cholesterol efflux (Li et al., 2016; Biswas et al., 2017). TSPO is also associated with other cellular functions such as oxidative stress, inflammation and apoptosis. Deletion of TSPO in rodents results in divergent characteristics possibly due to the methodology of creating the knockout models or the difference of genetic background between the knockout models (Li et al., 2016). Immunochemistry has shown TSPO is expressed at high levels in a human RPE cell line and the mouse RPE layer (Figure 1). Its expression is decreased in aged mouse neuroretina and RPE. TSPO is proposed to mediate cholesterol efflux in the RPE and loss of TSPO in RPE cells results in cholesterol efflux deficiency and intracellular accumulation of cholesterol (Biswas et al., 2017).