周围神经损伤
-
Figure 1|Design of various tubular constructs as nerve conduits.

Bridging and re-connecting a damaged nerve tissue is an important role of the neural scaffold. To achieve this goal, scaffolds which resemble the architecture of a nerve conduit were selected in earlier studies owing to their structural similarity to the tubular-like structures of nerves (Figure 1A and B). However, the hollow architecture can, potentially, lead to the axons extending through and along the internal surfaces of the conduit with only a few axons remaining in the centre of the tubular structure, which can limit the connectivity and nerve repair (Brushart et al., 1995; Yi et al., 2019; Ghane et al., 2021). Without an appropriate axon distribution across the whole width of the nerve conduit, there is a possibility of axon mismatching and, subsequently, disconnection in the target area (Gu et al., 2011).
The next generation of neural scaffolds that succeeded the earlier tubular model was the multi-channel nerve conduit comprised of multiple sub-micron-sized hollow tubes that were intended to mimic nerve fascicles within a nerve (Figure 1C). While this approach would have potentially resulted in a greater long-distance nerve regeneration compared to a single hollow conduit type, the effectiveness of the multichannel nerve conduit was found to be limited by its low permeability for the fluids to enter the interior of the multichannel structure and its high rigidity (De Ruiter et al., 2008; Yi et al., 2019).
Another approach that has been considered is to infuse nerve conduits with physical lumen fillers in which the filaments are embedded into the internal area of the nerve tubes (Figure 1D) allows the potential surfaces to grow on across the entire breadth of the conduit. The neurites growing through the filled lumen are able to retain their alignment, orientation and spatial arrangements, which reduce unwanted axon dispersions and increase the interactions with the distal nerves (Yi et al., 2019). To date, the ECM components such as collagen, laminin, fibrin, heparin and heparin sulphate, as well as micro- or nano-filaments coated and/or functionalized with the ECM components, are the most frequently used lumen fillers for this type of the conduit. These conduit fillers have been shown to promote neural growth in various models. Recent work by Entekhabi et al. (2021) employed collagen-hyaluronic acid sponge as a filler of an electrospun conduit to increase axonal growth in dorsal root ganglion cells.
The constructs with lumin fillers can also be loaded with cells prior to transplantation (Liu et al., 2021a; Onode et al., 2021). Debski et al. (2021) reported that a tubular-shaped structure made of electrospun fibres enriched with adipose-derived stem cells produce a high density of axons in a nerve gap of a rat sciatic nerve. Lee et al. (2021) suggested that transplanting olfactory ensheathing cells embedded in a nerve conduit in the form of a lumen filler can enhance the regeneration of injured PNS cells.
Despite the fact that the nanofibrous scaffolds themselves are quite promising for use as luminal fillers (Yi et al., 2019), these scaffolds with their own pseudo two dimensional geometries are still far from being able to offer a desired structure that is required for the ideal nerve conduits and as such, re-shaping fibrous sheets into a tubular design structure has been proposed as a possible solution. However, a possible damage to the sheet during the re-shaping process and a spontaneous folding of the sheet at the target site remain the main concerns if this approach to be implemented. The combination of additive fabrication technology with the application of ES methods may help to overcome the current challenges, and these technical solutions can lead to the next generation of truly 3D fibrous substrates, including carefully designed multilayer nerve scaffolds (Asadian et al., 2020; Liu et al., 2021b; Figure 1E).Figure 2|Schematic of the peripheral nerve components across an injury site.
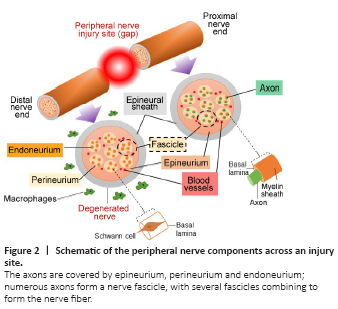
The ultimate aim of scaffold fabrication is to reproduce the features of the natural ECM (Watson et al., 2017). The ECM of the PNS displays a non-homogeneous 3D structure and an intricate architecture with complex components consisting of many different proteins and polysaccharides which influence the cellular response (Hernández et al., 2007; Soman and Vijayavenkataraman, 2020). In the PNS, the Schwann cells and axons are covered by epineurium, perineurium and endoneurium, and the ECM is located in the endoneurium and the basal lamina of the Schwann cells. The peripheral nerve components across the site of an injury are illustrated in Figure 2.