视神经损伤
-
Figure 1|Neuroprotective effects of siponimod on function and structure of the retina and optic nerve (ON) in high intraocular pressure (IOP) condition.
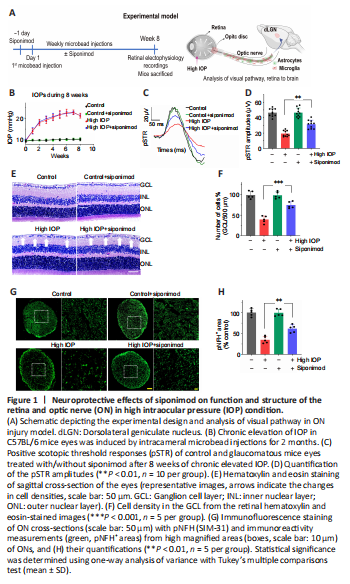
The potential protective effects of siponimod on inner retinal function and structure were measured in a high IOP mouse model (Figure 1A). For functional assessment of the inner retina, we measured the pSTR amplitudes (You et al., 2013). Siponimod-treated mice with intracameral injections of microbeads showed elevated IOPs comparable to the untreated control animals (Figure 1B). After 8 weeks, the high IOP subjected eyes exhibited a decline in pSTR amplitudes, while mice treated with siponimod (10 mg/kg in the diet and fed ad libitum) showed significant protection against the pSTR amplitude loss (P < 0.01; Figure 1C and D).
Assessment of cellular changes in the ganglion cell layer (GCL) and ON damage was then performed. Histological analysis of the retinal sections stained with H and E revealed a significant decrease in the number of cells in GCL in high IOP subjected untreated mice eyes compared with the control normal IOP (sham) mice eyes (P < 0.001). GCL loss was significantly attenuated in the siponimod-treated mice group compared with the untreated mice group in high IOP conditions, where GCL cell density loss decreased from 60.64 ± 8.73% to 25.29 ± 8.21% (P < 0.001; Figure 1E and F). Neurofilament heavy chain in RGC axons has been reported to undergo dephosphorylation in eyes subjected to elevated IOP (Kashiwagi et al., 2003; Chidlow et al., 2011). Immunofluorescence staining of ON cross-sections with phosphorylated neurofilament heavy chain (pNFH) antibody and pNFH+ area measurements revealed a significant decrease in pNFH immunoreactivity in high IOP ONs. This ON axonal damage in elevated IOP conditions was decreased from 64.88 ± 8.25% to 38.21 ± 9.15% with siponimod treatment compared with the untreated mice (P < 0.01; Figure 1G and H). These results indicated that siponimod exerts neuroprotective effects on the retina and ON against neurodegenerative changes induced by chronic high IOP.
Figure 2|Neuroprotective effects of siponimod on function and structure of the retina and optic nerve (ON) against acute retinal N-methyl-D-aspartate (NMDA) excitotoxicity.
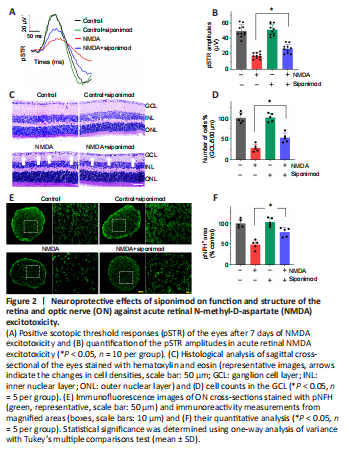
The neuroprotective effect of siponimod on the retina was further tested in an acute NMDA excitotoxicity model. A significant retinal functional deterioration in response to NMDA toxicity was observed after 7 days post-injury. The pSTR amplitudes in NMDA-treated mice were reduced by 65.33 ± 3.78%, and this reduction was significantly protected with siponimod treatment (P < 0.05; Figure 2A and B). H and E staining of retinal sections and examination of ONs stained with pNFH further revealed the protective effects of siponimod. NMDA-induced cell loss in GCL decreased from 71.36 ± 9.61% to 47.58 ± 11.50% in the siponimod treated group compared with the untreated mice group (P < 0.05; Figure 2C and D). Axonal damage assessed by pNFH+ area measurements showed pNFH immunoreactivity loss was decreased from 51.39 ± 10.99% to 22.02 ± 10.74% in siponimod treated mice in NMDA excitotoxicity condition compared with the untreated mice group (P < 0.05; Figure 2E and F). These results emphasize the protective effects of siponimod treatment on the retina in the NMDA injury model.
Figure 3|Protective effects of siponimod on the dorsolateral geniculate nucleus (dLGN) of the brain in chronic glaucoma condition (8 weeks).
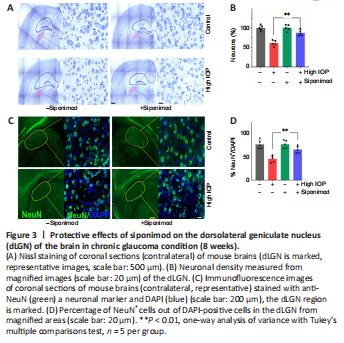
Neuronal degenerative changes in the dLGN have been documented in ON injury conditions (Gupta et al., 2007; Yücel and Gupta, 2008; Sriram et al., 2012). The effect of siponimod on trans-neuronal degeneration under chronic elevated IOP in the dLGN region of the brain was evaluated by staining coronal brain sections with Nissl blue and NeuN. Quantitative neuronal cell density analysis revealed a significant protective effect of siponimod on neuronal cell loss in the dLGN. A decline of 38.95 ± 7.26% in neuronal cell density was observed in high IOP mice, and this degeneration was reduced to 12.53 ± 7.85% (P < 0.01) in the siponimod treated group compared with the untreated mice group (Figure 3A and B). Further, in high IOP eyes, the frequency of NeuN+ cells was decreased by 39.34 ± 6.77%, whereas this loss was reduced to only 13.72 ± 7.36% in the siponimod treated group (Figure 3C and D). The higher visual cortex (V1) analysis in this high IOP condition for 2 months period showed no significant trans-neuronal degenerative changes. These results together establish a significant protective effect of siponimod on the neuronal cell population in the dLGN region of the brain.
Figure 5| Siponimod treatment reduces glial activation along the visual pathway in chronic optic nerve injury condition (8 weeks).
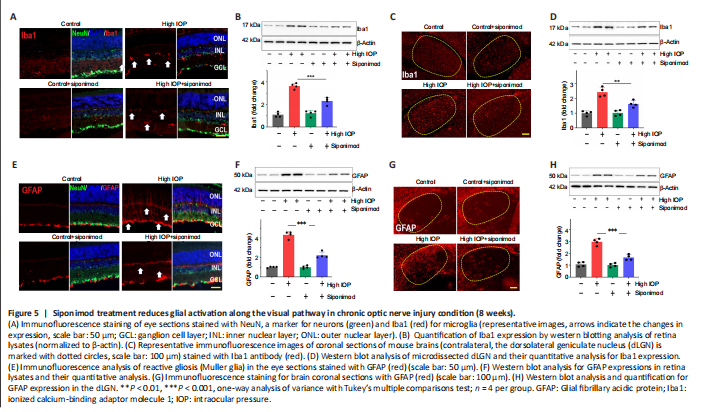
Chronic neuroinflammation in glaucoma is implicated as an important component in disease pathogenesis. The early reactivity of glial cells (microglial activation and reactive gliosis) has been observed in the retina (Mélik Parsadaniantz et al., 2020; Rolle et al., 2020). Glial activation along the visual pathway was evaluated by investigating Iba1 and GFAP expression alterations. Immunofluorescence staining of the retina, ON, and the brain tissue sections with Iba1 and GFAP revealed activation of glial cells along the visual pathway in chronic high IOP condition (Figure 5 and Additional Figure 1). Activated microglia in the retina, ON, and dLGN were reduced with siponimod treatment in chronic high IOP conditions (Figure 5A, C and Additional Figure 1A). Microglial activation in the ON evaluated by measuring changes in Iba1+ areas indicated a significant increase (3.02 ± 0.29-fold) of Iba1 immunoreactivity in the high IOP model. This activation was reduced in the siponimod-treated group to 1.84 ± 0.41 fold (Additional Figure 1A). Western blot analysis of retinal and dLGN tissues for Iba1 expression revealed a 3.63 ± 0.25-fold increase in the retina and 2.43 ± 0.32-fold increase in dLGN in high IOP conditions, and this upregulation was significantly diminished with siponimod treatment (2.27 ± 0.31-fold in retina and 1.63 ± 0.22-fold in dLGN) (Figure 5B and D).
In control retinas, GFAP immunoreactivity was primarily confined to the GCL. High IOP retinas showed GFAP labeling as a dense network of Müller cell processes in the GCL along with the emergence of a radial pattern of GFAP immunopositivity (Figure 5E). Similar hypertrophic reactive astrogliosis with upregulated GFAP was observed in ON (Additional Figure 1B) and dLGN of high IOP subjected mice (Figure 5G). This reactive gliosis was diminished with siponimod treatment. Western blot quantitative analysis of GFAP expression in the retina and dLGN tissues showed 4.33 ± 0.35-fold and 2.96 ± 0.27-fold upregulation, respectively in high IOP conditions compared with the control normal IOP (sham) mice group. Siponimod treatment resulted in its suppression to 2.23 ± 0.31-fold in the retina and 1.65 ± 0.23-fold in dLGN compared with the untreated mice (Figure 5F and H). GFAP immunoreactivity in the ON was increased to 3.47 ± 0.5-fold in high IOP condition compared with the control normal IOP (sham) mice but was reduced significantly to 1.96 ± 0.30 fold in siponimod treated mice (P < 0.001; Additional Figure 1B). These results indicated that the siponimod treatment effectively reduced microglial activation and reactive gliosis in chronic high IOP experimental glaucoma.
Figure 6|The functional importance of S1PR1 in neurons and its deletion impairs the protective effect of siponimod on inner retinal function and structure in chronic optic nerve injury condition.
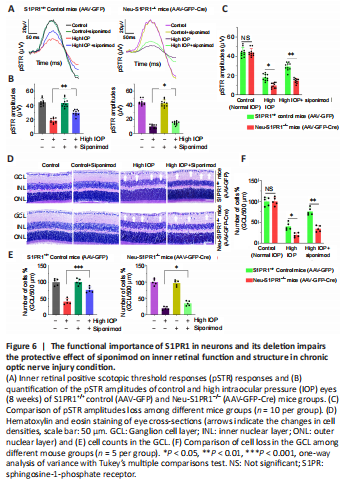
The mice injected with AAV-GFP referred to as S1PR1+/+ control mice (AAV-GFP) and the neuron-specific S1PR1 deleted mice (termed as Neu-S1PR–/– mice (AAV-GFP-Cre)) were administered siponimod diet (Additional Figure 3). No significant functional or structural changes were observed in the retina upon neuronal S1PR1 ablation under normal conditions. However, upon microbead-induced ON injury, neuronal S1PR1 ablated mice showed a significant decrease in the pSTR amplitudes and GCL thinning. Interestingly, the protective effects of siponimod were significantly attenuated in the group subjected to neuronal S1PR1 impairment (Neu-S1PR–/–, AAV-GFP-Cre) compared with control S1PR+/+ animals (AAV-GFP). High IOP-induced pSTR amplitude loss of 57.69 ± 5.03% was reduced to 32.58 ± 4.57% in siponimod-treated S1PR+/+ control animals. However, NeuN-S1PR1–/– animals exhibited 77.62 ± 2.74% loss in high IOP eyes compared with 66.43 ± 2.45% loss in the siponimod treated group (P < 0.01; Figure 6A–C).
Further, quantitative analysis of the GCL from H and E stained retinal sections revealed a significant decrease (P < 0.05) in GCL density in Neu-S1PR1–/– ablated (AAV-GFP-Cre) mice compared with S1PR1+/+ control (AAV-GFP) mice in high IOP conditions. The siponimod-mediated protective effect was significantly diminished in Neu-S1PR1–/– ablated mice compared with control S1PR1+/+ animals (P < 0.01; Figure 6D–F). ON damage was assessed by performing pNFH immunoreactivity measurements that showed similar changes between Neu-S1PR1–/– ablated mice and S1PR1+/+ control mice. However, the siponimod-treated group showed significantly greater immunoreactivity of pNFH only in S1PR1+/+ control mice compared with Neu-S1PR1–/– mice (P < 0.01; Additional Figure 4).
Figure 7|Neuron-specific deletion of S1PR1 reduced the protective effect of siponimod against trans-neuronal degeneration in dLGN of the brain in chronic optic nerve injury condition.
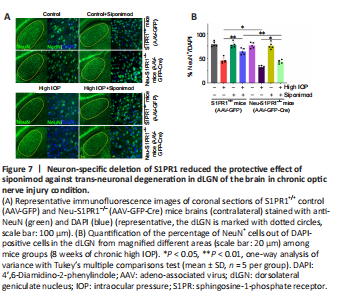
We next analyzed the impact of neuronal S1PR1 ablation on degenerative changes in dLGN of the brain. A significant decline in the frequency of NeuN+ cells was observed in neuronal S1PR1 ablated mice (AAV-GFP-Cre) compared with control S1PR1+/+ (AAV-GFP) mice in high IOP conditions (P < 0.05). No significant changes in dLGN neuronal cell density were evident in animals subjected to neuronal S1PR1 ablation under normal conditions. Neuronal S1PR1 ablation significantly reduced the protective effects of siponimod on the dLGN degenerative changes. Siponimod treatment showed a significantly greater frequency of NeuN+ cells in S1PR+/+ control animals compared with Neu-S1PR1–/– mice (P < 0.01) in high IOP conditions (Figure 7). Quantitative histological analysis of the neuronal cell density showed a significantly reduced neuronal density (P < 0.01) in the dLGN region in Neu-S1PR1–/– ablated mice compared with the control S1PR1+/+ animals in high IOP conditions. The protective effect of siponimod on the dLGN neuronal density observed in the control S1PR1+/+ mice was significantly diminished in the Neu-S1PR1–/– ablated group under high IOP conditions (P < 0.01; Additional Figure 5). These results indicated that expression of S1PR1 in the neurons plays a pivotal role in cell survival in the retina and dLGN in high IOP-induced degeneration and that the protective effects of siponimod on the neurons under such conditions are mediated through neuronal S1PR1 expression.
Figure 8|Attenuation of the protective effect of siponimod on retinal microglial activation and reactive Müller glia in high IOP condition in Neu-S1PR1–/– (AAV-GFP-Cre) mice.
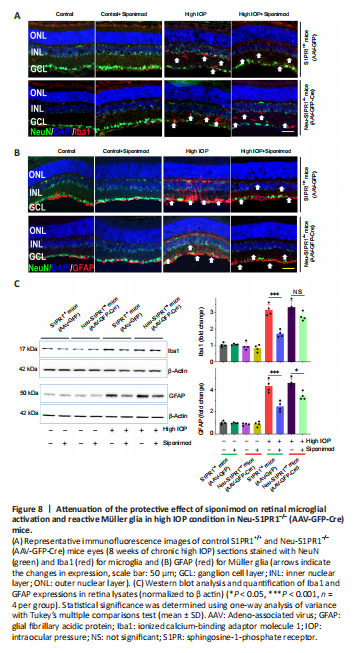
We then determined whether the increased degeneration observed in Neu-S1PR1–/– (AAV-GFP-Cre) mice with high IOP might impact siponimod-induced effects on glial activation. Similar to the results described in Figure 5 and Additional Figure 1, hypertrophic activated microglia and reactive gliosis analyzed with Iba1 and GFAP antibodies along the visual pathway were observed in untreated control S1PR1+/+ (AAV-GFP) mice in chronic high IOP conditions and this was reduced with siponimod treatment (P < 0.001; Figure 8 and Additional Figures 7 and 8). Western blot quantification revealed a 3.16 ± 0.35-fold increase in Iba1 and 4.30 ± 0.57-fold upregulation of GFAP expression in the retina (P < 0.001) in high IOP conditions compared with the control normal IOP (sham) mice group (Figure 8C). Similarly, in the dLGN 2.57 ± 0.28-fold increase for Iba1 and 3.17 ± 0.22-fold increase for GFAP expression were observed with chronic high IOP (P < 0.001; Additional Figure 7C). This glial activation was reduced significantly to 1.52 ± 0.18 (dLGN) to 1.69 ± 0.21-fold (retina) for microglia and 2.13 ± 0.27 (dLGN)- to 2.47 ± 0.37-fold (retina) for reactive gliosis with siponimod treatment in S1PR1+/+ control mice. This protective effect of siponimod on microglial activation was abolished in Neu-S1PR1–/– mice. However, siponimod treatment showed a significant suppressive effect on reactive gliosis in Neu-S1PR1–/– mice (AAV-GFP-Cre). Siponimod treatment significantly (P < 0.05) reduced GFAP expression from 4.57 ± 0.44 to 3.41 ± 0.35-fold in the retina and 3.72 ± 0.32 to 2.88 ± 0.22-fold in dLGN of the brain in Neu-S1PR1–/– mice under high IOP conditions compared with the untreated mice group. Further, similar levels of effects were observed in ONs evaluated by immunoreactivity of Iba1 and GFAP (Additional Figure 8). These results together suggest that a diminished protective effect of siponimod on glia was associated with enhanced degeneration after S1PR1 deletion in neurons.