脊髓损伤
-
Figure 3|NC-MS treatment attenuates the astrocyte activation in rat models of incomplete SCI.
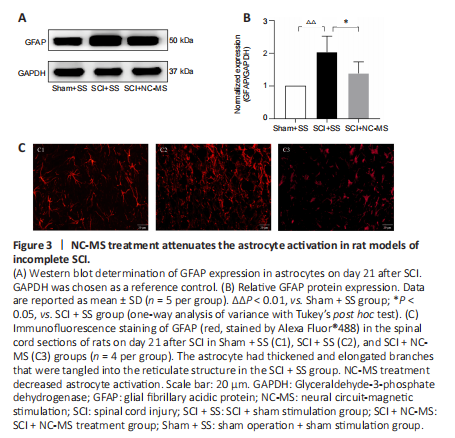
To assess the effect of NC-MS treatment on astrocyte activation in incomplete SCI, we performed western blot assays and immunofluorescence staining in three groups on day 21 after SCI to determine the degree to which the astrocyte-specific marker GFAP was expressed. Western blot results showed that GFAP expression was significantly higher in the SCI groups than in the Sham + SS group (P < 0.01; Figure 3A and B). Among the two SCI groups, GFAP expression was significantly lower in the SCI + NC-MS treatment group than in the SCI + SS group (P < 0.05). Immunofluorescence staining yielded similar results (Figure 3C). On day 21 after SCI, we observed high GFAP expression around the injured area as well as activated astrocytes that were swollen and hypertrophic. The active astrocytes exhibited thickened and elongated branches that were tangled into the reticulate structure, forming a dense and irregular glial scar. However, compared with the SCI + SS group, NC-MS treatment resulted in less GFAP expression and a smaller degree of swelling and astrocyte activation. These results indicate that NC-MS treatment attenuated astrocyte activation and reduced glial scar formation.
Figure 5|NC-MS treatment enhances the neural survival and repair, and synaptic plasticity in rat models of incomplete SCI.
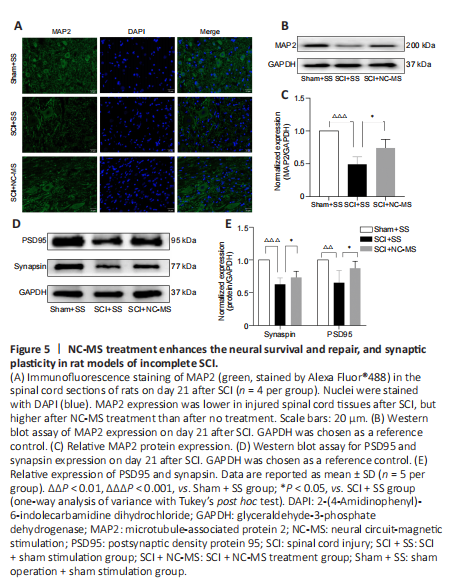
TMS can promote neural repair and synaptic plasticity in many neurological disorders (Iglesias, 2020). Hence, we performed immunofluorescence staining and western blot assay to confirm whether NC-MS treatment can have the same effect after SCI. Immunofluorescence staining (Figure 5A) and western blot (Figure 5B and C) analysis showed that MAP2 expression was lower in injured spinal cord tissue after SCI. However, significantly higher MAP2 levels were observed in the SCI + NC-MS group than in the SCI + SS group (P < 0.05; Figure 5C). Furthermore, we explored the influence of NC-MS on PSD95 and synapsin expression, both of which are related to synaptic plasticity. Western blot data suggested that NC-MS treatment leads to significantly more up-regulation of synaptic plasticity-related proteins than occurs without stimulation (P < 0.05; Figure 5D and E). Thus, these findings confirmed that NC-MS treatment can enhance factors related to neural survival, repair, and synaptic plasticity in rat models of incomplete SCI.