周围神经损伤
-
Figure 2|Proliferation of astrocytes and Schwann cells following treatment with GDNF and BDNF.
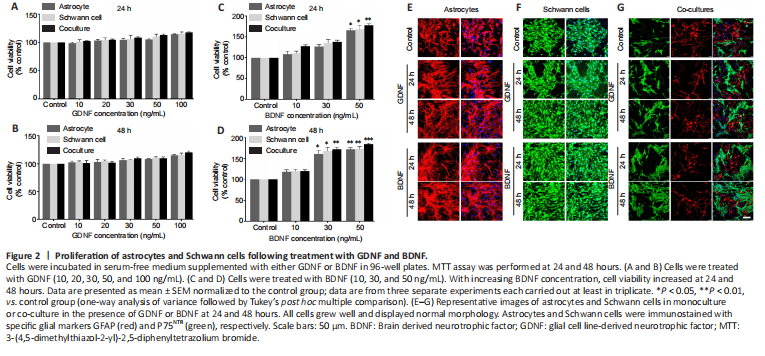
To investigate the effects of GDNF and BDNF on astrocytes and Schwann cell growth and survival, cells in monoculture were cultured with various concentrations of GDNF (10, 20, 30, 50 and 100 ng/mL) and BDNF (10, 30 and 50 ng/mL) for 24 and 48 hours. Cell viability was assessed by MTT assay. Treatment with GDNF at all concentrations for 24 and 48 hours did not result in significant changes in viability compared with the control group (Figure 2A and B). In contrast, treatment with increasing doses of BDNF resulted in increased viability at 24 hours (Figure 2C) and 48 hours (Figure 2D). Immunostaining for GFAP (astrocytes) and P75NTR (Schwann cell) confirmed that astrocytes and Schwann cells in monoculture and co-culture grew well with normal morphology in the presence of GDNF (50 ng/mL) or BDNF (50 ng/mL) at 24 and 48 hours (Figure 2E–G).
Figure 3|The effect of GDNF and BDNF on the migration of AS and SC in the confrontation assay.
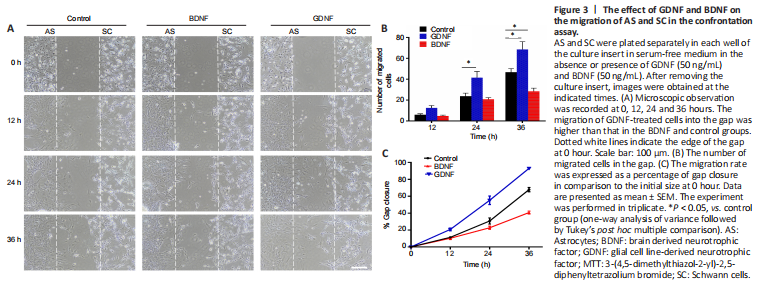
The results showed that GDNF facilitated cell migration into the cell free-gap and increased the migration rate of both cell populations towards each other, resulting in a higher number of migrating cells compared with the control group (Figure 3A and B). In contrast, BDNF markedly reduced the number of migrating cells compared with the control group (Figure 3A and B). The migration rate of GDNF-treated cells was higher than that in the BDNF and control groups (Figure 3C). This showed that GDNF enhanced cell motility.
Figure 4|The intermingling between astrocytes and Schwann cells on the boundary assay.
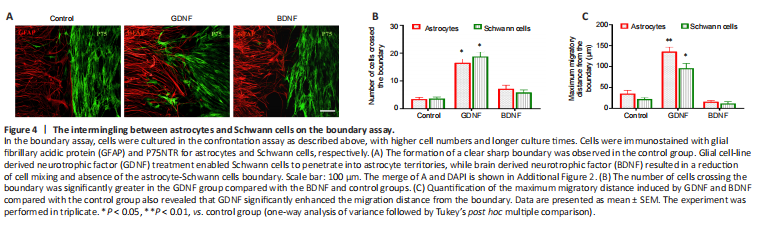
To assess the influence of GDNF and BDNF on cell boundary formation, the confrontation assay was repeated using higher number of cells for longer periods of time. In the control group, as cells migrated towards one another, a boundary between the two cell types were clearly visible (Figure 4A–C). In contrast, the inhibited cell migration prevented full establishment of boundary formation in the BDNF group. Cells in the GDNF group showed increased migration, and Schwann cells were observed to penetrate into the area of the cultured astrocytes.
Figure 5|Migration assay of Schwann cell monoculture.
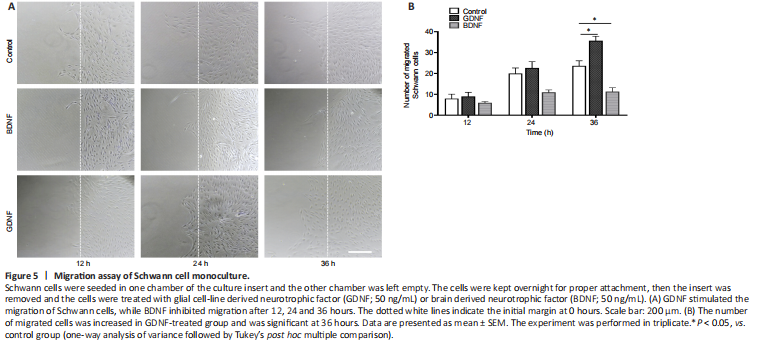
In the experiments described above, we did not distinguish whether GDNF or BDNF acts directly on astrocytes or Schwann cells to induce migration. To address this question, Schwann cells or astrocytes were each plated in one chamber of the culture insert and the other chamber was left empty. We found that administration of GDNF induced migration of Schwann cells to the cell-free gap (Figure 5A and B). There was no remarkable effect on astrocyte monoculture (data not shown).
Figure 6|The morphological transformation of primary astrocytes in vitro.
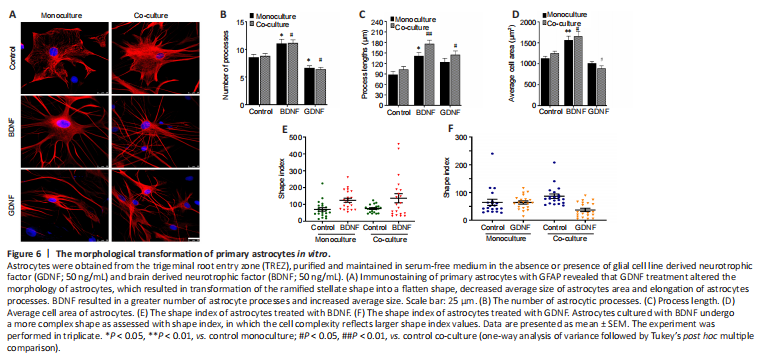
Staining of primary astrocytes with GFAP revealed that GDNF treatment altered the morphology of astrocytes, with transformation of the ramified stellate shape into a flatten shape, a decreased average size of astrocyte area and elongated astrocyte processes (Figure 6A). Furthermore, the GDNF-treated cells showed two or three very long processes. The morphometric changes were more commonly observed in co-cultured astrocytes than in monocultured astrocytes. In contrast, administration of BDNF to astrocytes cultured alone or in co-culture induced an increase in the average cell area, enhancement of the number and length of astrocytes processes (Figure 6B–D). The processes of BDNF-treated cells were very fine, straight and relatively longer than those of GDNF-treated cells. This finding indicates that astrocytes cultured with BDNF transform into a more complex shape as assessed by calculating shape index (Figure 6E and F). Notably, the cell soma area was relatively increased together with the number of primary processes emerging from the cell body. These findings indicate that GDNF and BDNF are potent regulators of the morphology of astrocytes in the TREZ.
Figure 7|Expression of glial fibrillary acidic protein (GFAP) and P75NTR in astrocytes and Schwann cells, respectively.
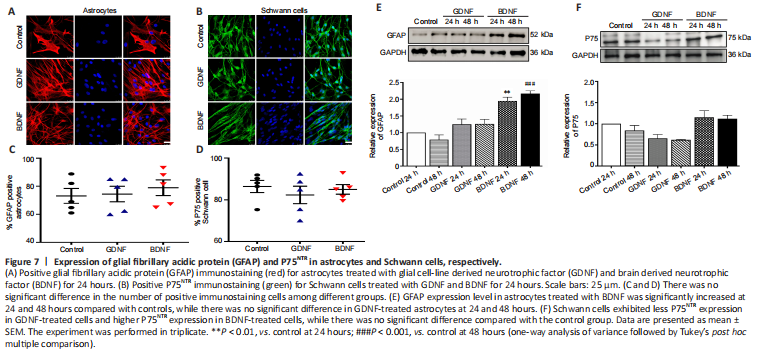
Immunostaining of GFAP and P75NTR for astrocytes and Schwann cells showed there was no significant difference in the number of positive immunostained cells among different groups (Figure 7A–D). To verify the effects of GDNF or BDNF on cultured cells from TREZ, western blotting was performed in astrocytes and Schwann cells. The results showed that GFAP expression level in astrocytes treated with BDNF was higher than that of the control group at 24 hours and was much greater at 48 hours (Figure 7E). There was no significant difference in expression level of GFAP in GDNF-treated astrocytes. Schwann cells exhibited less P75NTR expression in GDNF-treated cells and high P75NTR expression in BDNF-treated cells (Figure 7F).