脑损伤
-
Figure 1|Fibrotic scar formation occurs in the ischemic core after middle cerebral artery occlusion/reperfusion (MCAO/R) in rats.
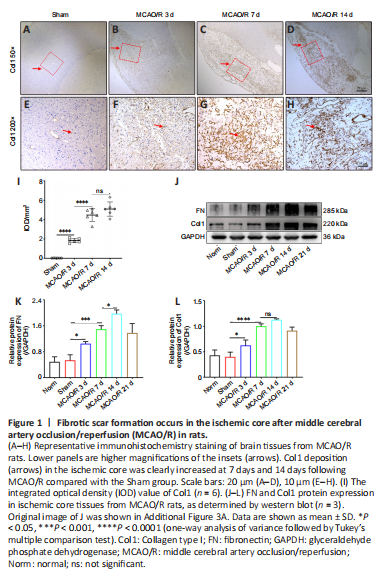
The fibrotic scar that forms after tissue injury mostly consists of myofibroblasts and excess ECM, primarily containing collagen (Col), fibronectin (FN), and laminin (Li et al., 2021b). Therefore, we first evaluated whether MCAO/R injury induced the formation of fibrotic scar within the ischemic core in rats. Immunohistochemistry staining showed that Col1 (Figure 1A–I) deposition in the ischemic core was increased at 7 and 14 days following MCAO/R compared with the Sham group. Moreover, western blot analysis demonstrated that FN and Col1 expression were higher in the MCAO/R group than in the Norm and Sham groups (Figure 1J–L), peaked at 14 days, and then gradually decreased. Taken together, these results show that MCAO/R injury induces significant fibrosis within the ischemic core in rats.
Figure 2|Macrophages infiltrate into the ischemic area and may be positively correlated with fibrotic scar formation.
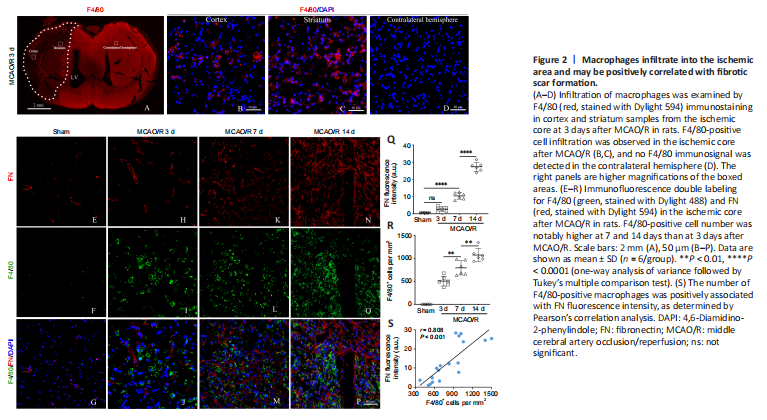
F4/80 is a monocyte-derived macrophage marker that has relatively high specificity (Wang et al., 2020). We first determined the temporal and dynamic response of macrophages after MCAO/R injury. As revealed by immunofluorescence staining, excessive F4/80-positive cell infiltration was observed in the ischemic core 3 days after MCAO/R, which was not seen in the contralateral hemisphere or in the Sham group (Figure 2A–D). In addition, the number of F4/80-positive cells was notably higher in the ischemic core at 7 and 14 days than that at 3 days after MCAO/R, and peaked at 14 days (Figure 2E–P). F4/80-positive cells had a uniform round shape without apparent processes. Immunofluorescence double staining demonstrated that FN-positive fibers and F4/80-positive macrophages were co-localized, and this interaction became more pronounced over the time course of injury (Figure 2E–R). Pearson’s correlation analysis suggested a robust positive association between the number of F4/80-positive macrophages and FN fluorescence intensity (Figure 2S).
Figure 3|Macrophage depletion prevents fibrotic scar formation in the lesion core after MCAO/R in rats.

Next, circulating monocyte/macrophages were transiently depleted with clodronate-filled liposomes (Figure 3A), which mediate macrophage “suicide” (Miró-Mur et al., 2016). After Clo treatment for 7 days following cerebral ischemia in rats, the number of F4/80-positive cells in the ischemic core was two-fold lower than that seen in mice that received vehicle-filled liposomes (Veh) (Figure 3B–I). At the same time, The number of FN-positive fibers and Col1-positive fibers decreased by 65.3% and 41.3%, respectively, in the Clo group compared with the Veh group at 7 days post-MCAO/R in rats (Figure 3J–O). In addition, western blot analysis showed that FN and Col1 expression was reduced in the Clo group compared with the Veh group at 7 days post-MCAO/R in rats (Figure 3P and Q).
Figure 4|Macrophages undergo M2 polarization in the ischemic core and in vitro after IL4 treatment.

First, we determined the concentrations of IL4/6/10 and IFNγ in the ischemic core post-MCAO/R. We found that IL4 expression increased within 3 days following cerebral ischemia, remained steady for at least 14 days, and then decreased dramatically at 21 days (Figure 4A), while IL10 expression increased later, at 14 days post-MCAO/R (Figure 4B). Expression of the pro-inflammatory cytokines IFNγ and IL6 increased shortly and slightly after MCAO/R (Figure 4C and D). In addition, greater numbers of F4/80-positive and CD206-positive (an M2 macrophage marker) cells were found in the ischemic core in the IL4-treated rats compared with the PBS-treated rats (Figure 4E–K) at 7 days after MCAO/R. Furthermore, stimulating RAW264.7 cells (Figure 4L and M) or BMDMs (Figure 4N and O) with recombinant rat IL4 for 2 days in vitro resulted in a clear increase in the number of CD206-positive cells.
Figure 5|MCM promotes fibroblast proliferation and activation.

Figure 6|RCM induces extracellular matrix production by fibroblasts.
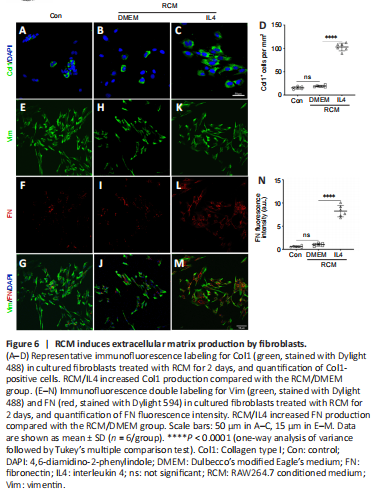
Fibroblasts are vital to ECM remodeling and deposition. Macrophages form a stable association with fibroblasts and exert reciprocal control of cell survival and proliferation (Zhou et al., 2018). To investigate whether fibroblasts are directly related to macrophages, we analyzed how the MCM or RAW264.7-conditioned medium (RCM) affected cultivated fibroblasts (Figure 5A). According to the EdU cell proliferation assay, immunofluorescence staining, and western blot assay results, MCM/DMEM increased the number of EdU-positive proliferating fibroblasts (Figure 5B–E), α-smooth muscle actin-positive myofibroblasts (Figure 5F–I), and Col1-positive fibroblasts (Figure 5J–M), as well as the FN fluorescence intensity of fibroblasts (Figure 5N–W) and the production of Col1 and FN (Figure 5X and Y) compared with the control group, and MCM/IL4 further enhanced these effects. Moreover, immunofluorescence staining showed that RCM/IL4 increased Col1 and FN production to a greater extent than RCM/DMEM (Figure 6).
Figure 7|IL4 treatment induces macrophages to undergo M2 polarization and produce extracellular matrix in the ischemic core.

Next, we investigated the effects of IL4 on fibrogenesis and macrophage M2 polarization after MCAO/R in vivo. Based on western blot assay and immunofluorescence or immunohistochemistry staining, both Col1 and FN deposition and the number of CD206-positive macrophages were clearly elevated in the IL4-treated groups than in the PBS-treated groups at 7 days after MCAO/R (Figure 7A–H). Pearson’s correlation analysis demonstrated a robust positive correlation between the number of CD206-positive macrophages and FN fluorescence intensity (Figure 7I). Furthermore, western blotting showed that Col1 and FN protein levels in the ischemic core were higher in the IL4-treated groups at 14 days after MCAO/R than at 7 days after MCAO/R (Figure 7J–O). These results suggest that IL4-induced M2 macrophages participate in fibrogenesis after MCAO/R.
Figure 8|IL4-induced M2 macrophages secrete Shh and activate Shh signaling in the ischemic core and in vitro.
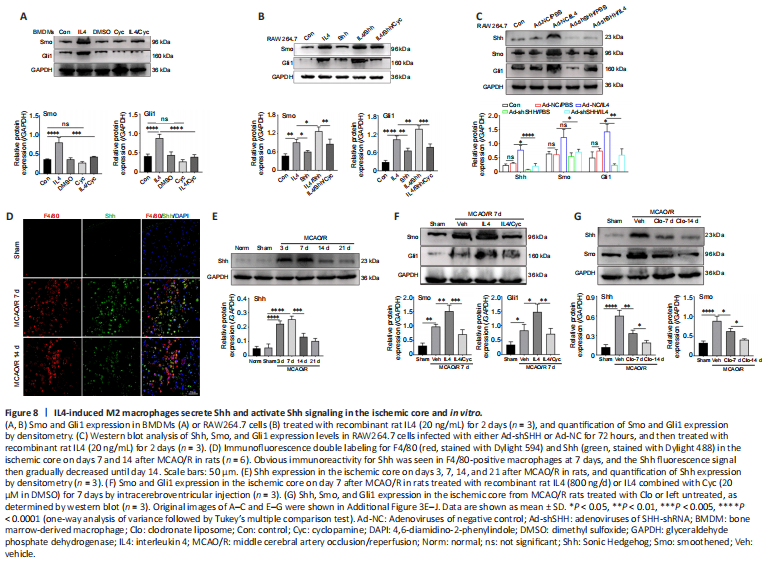
In BMDM cells, IL4 treatment enhanced Smo and Gli1 expression, and treatment with the Smo receptor inhibitor Cyc inhibited Smo and Gli1 expression (Figure 8A). However, treatment of RAW264.7 cells with Shh alone had little effect on Gli1 and Smo expression levels. Combined treatment with IL4 and Shh markedly elevated Gli1 and Smo expression levels compared with treatment with Shh alone or IL4 alone, and combined treatment with IL4, Shh, and Cyc markedly reduced Gli1 and Smo expression levels (Figure 8B). Additionally, knockdown of Shh in Raw264.7 cells abrogated the effects of IL4 on Gli1, Shh, and Smo expression levels (Figure 8C).
Following cerebral ischemia, clear immunoreactivity for Shh could be seen in F4/80-positive macrophages at 7 days, and the Shh fluorescence signal gradually decreased until day 14 (Figure 8D). Shh expression in the ischemic core was markedly increased as early as day 3, peaked on day 7, and then declined, as determined by western blot assay (Figure 8E). Further, Smo and Gli1 expression levels in the ischemic core were notably increased at 7 days after ischemia in rats treated with recombinant rat IL4 by intracerebroventricular injection compared with the Sham and Veh groups; and this effect was abrogated by combined treatment with IL4 and Cyc (Figure 8F). Shh and Smo expression levels in the ischemic core following cerebral ischemia were notably reduced in rats treated with Clo for 7 days compared with the Veh group, and were further lowered by treatment with Clo for 14 days (Figure 8G). These results suggest that IL4-induced M2 macrophages maybe secrete Shh and activate the Shh pathway in vivo and in vitro.
Figure 9|Shh signaling regulates extracellular matrix protein production.

Next, we explored whether the Shh signaling pathway affected the production of FN and Col1 in fibroblasts treated with MCM in vitro and in the MCAO/R model in vivo. Western blot analysis demonstrated that FN and Col1 expression in fibroblasts were higher in the MCM/IL4 group than in the MCM/DMEM, Con, or DMEM/IL4/Cyc groups, and that their expression was abolished by MCM/IL4/Cyc (Figure 9A). In vivo, Shh knockdown decreased FN and Col1 expression levels in the ischemic core at 7 days after MCAO/R (Figure 9B and C) and abrogated the effects of IL4 on FN and Col1 expression (Figure 9C). Furthermore, Shh overexpression had the opposite effect on FN and Col1 expression (Figure 9D and E). These results suggest that Shh signaling regulates FN and Col1 expression in vitro and in vivo.
Figure 10|IL4-mediated Shh signaling regulates TGFβ1 and MMP9 expression.
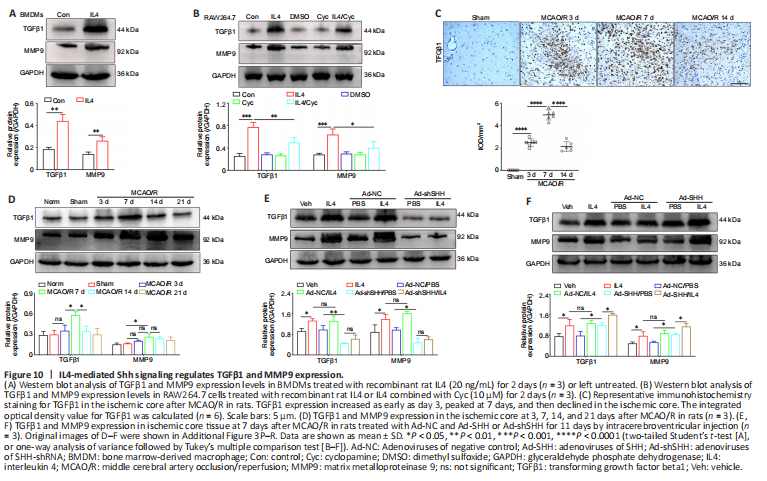
Stimulating BMDMs with recombinant rat IL4 for 2 days markedly increased MMP9 and TGFβ1 expression levels compared with the control group (Figure 10A), and MMP9 and TGFβ1 expression in RAW264.7 cells was clearly inhibited by treatment with the Smo receptor inhibitor Cyc or both IL4 and Cyc (Figure 10B).
TGFβ1 and MMP9 expression in rats in the ischemic core increased as early as day 3 after MCAO/R, peaked on day 7, and then declined; furthermore, the expression of both proteins was notably reduced by Shh knockdown and enhanced by Shh overexpression on day 7 (Figure 10C–F). Moreover, Shh knockdown and overexpression respectively abrogated and enhanced the ability of IL4 to upregulate of TGFβ1 and MMP9 expression (Figure 10E and F). These results suggest that IL4-mediated Shh signaling regulates TGFβ1 and MMP9 expression in M2 macrophages in vitro and in the MCAO/R model in vivo.
Figure 11|Fibrotic scar formation is positively correlated with neovascularization induced by IL4-mediated Shh signaling.

Figure 12|IL4-mediated Shh signaling decreases infarct volume, apoptosis, and mNSS score after MCAO/R in rats.

Immunofluorescence staining showed that IL4 treatment increased the expression of CD31, an angiogenesis-related marker, in the ischemic core compared with the Control and Sham groups at 7 days after MCAO/R, and that this effect was blunted by treatment with Cyc or with both IL4 and Cyc. Moreover, the increase in CD31 expression was minimal in rats treated with Cyc alone (Figure 11A). Further, immunofluorescence double labeling demonstrated that FN fluorescence intensity was positively correlated with microvessel density (Figure 11B). In addition, TUNEL staining (Figure 12A–Q), TTC staining (Figure 12R and S), and modified neurological severity score (Figure 12T) indicated that IL4 treatment clearly decreased apoptosis and infarct volume and alleviated the neurological deficits in comparison with the control group, while these effects were abolished by treatment with Cyc or combined treatment with IL4 and Cyc, and were minimal in rats treated with Cyc alone. These results show that IL4-mediated Shh signaling promotes angiogenesis and decreases injury in the early stage of recovery after MCAO/R and may be associated with fibrotic scar formation.