脊髓损伤
-
Figure 1|Spinal surgery increases SP expression in the injured tissues.

To investigate the relationship between SP and epidural fibrosis, peripheral blood was obtained from patients before and 2 days after laminectomy. The SP content in postoperative blood samples was clearly higher than that in preoperative blood samples (Figure 1A), indicating that laminectomy may cause massive SP secretion. To verify this, we established a mouse model of spinal surgery (Figure 1B). Laminectomy caused injury to tissues surrounding the surgical area, including the spinal cord, muscles, and vertebral plates. Compared with the sham group, more SP-positive cells were observed at the wound site in the operated mice via histochemical staining (Figure 1C) and immunofluorescence staining (Figure 1D). Furthermore, increased SP expression was detected in the injured tissues by ELISA (Figure 1E). Collectively, these results suggest that spinal surgery increases SP expression in the injured tissues.
Figure 2| Dorsal root ganglion (DRG) neurons and macrophages produce SP.
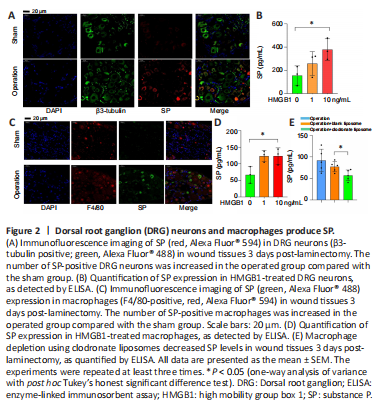
DRG neurons are recognized as SP-producing cells (Chen et al., 2014). SP was detected in the DRG neurons (β3-tubulin-positive cells; Deng et al., 2021) isolated from epidural wound tissues harvested from mice that underwent laminectomy (Figure 2A). In vitro, we stimulated DRG neurons with HMGB1, a multifunctional protein that can induce inflammation (Lotze and Tracey, 2005). The results showed that HMGB1 promoted SP secretion in DRG neurons (Figure 2B). In addition to DRG neurons, an increasing number of studies have shown that many non-nerve cells also secrete SP, including immune cells (Germonpre et al., 1999), smooth muscle cells (Warner et al., 2000), endothelial cells (Esteban et al., 2021), and airway epithelial cells (Esteban et al., 2009). Therefore, we investigated whether a portion of the SP at the wound site after laminectomy was derived from macrophages. At the wound site, we observed more macrophages that were doubly positive for F4/80 and SP in the operated group compared with the sham group (Figure 2C). Similarly, when HMGB1 was used to stimulate macrophages, the level of SP increased in the macrophage growth medium (Figure 2D). After using clodronate liposomes (Tian et al., 2017) to remove all macrophages in mice that underwent laminectomy, we observed a decrease in SP expression at the wound site, while the mice injected with blank liposomes did not show a decrease in SP in the epidural wound area (Figure 2E), indicating that macrophages play an important role in SP production in epidural wound tissues after spinal surgery. In summary, the SP that is present in epidural wound tissues post-spinal surgery may be produced by DRG neurons and macrophages.
Figure 5| SP regulates metabolism in macrophages.
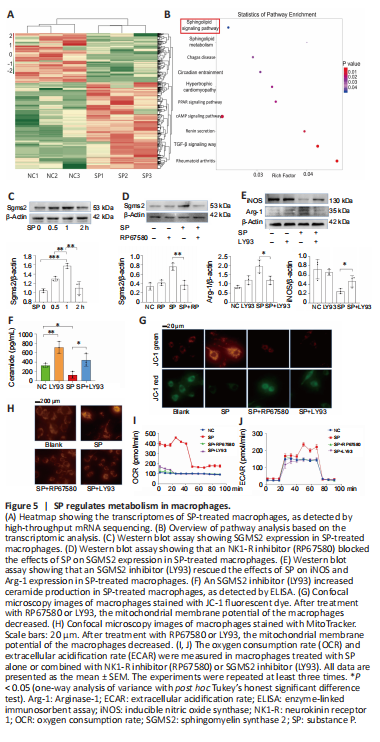
To explore the intracellular signaling changes in macrophages stimulated by exogenous SP, we conducted high-throughput bulk RNA sequencing of macrophages treated with SP or left untreated. Analysis of the differentially expressed genes (Figure 5A) and KEGG signaling pathway analysis (Figure 5B) highlighted the sphingolipid metabolic pathway as possibly being linked to macrophage differentiation. Sphingomyelin synthase 2 (SGMS2) is an essential component of the sphingolipid metabolic pathway (Pekkinen et al., 2019). Similar to the bulk-RNA seq results, western blotting confirmed that SP increased SGMS2 expression in macrophages (Figure 5C); furthermore, RP67580 blocked the effects of SP on SGMS2 expression levels (Figure 5D). To verify that SGMS2 is involved in SP-induced M2 differentiation, LY93, an inhibitor of SGMS2, was used (Li et al., 2019). The results showed that Arg-1, an M2 marker, was decreased, and iNOS, an M1 marker, was increased when SGMS2 was inhibited by LY93, suggesting that M2 differentiation in the SP-treated macrophages was largely suppressed (Figure 5E). Collectively, these results suggest that SMGM2 plays a key role in the SP-mediated promotion of M2 differentiation.
SGMS2 is an enzyme that regulates ceramide and sphingomyelin levels (Ou et al., 2021). As expected, the ELISA results showed that an increase in SGMS2 expression caused a decrease in the concentration of ceramide (Figure 5F), a type of phospholipid that causes substantial damage to the mitochondria (Dany et al., 2016; Dadsena et al., 2019). Therefore, we examined mitochondrial function in SP-treated macrophages. Mitochondrial membrane potential was detected using JC-1 dye (Figure 5G) and MitoTracker Red dye (Figure 5H). Blocking SP with RP67580 or blocking SGMS2 with LY93 suppressed the effects of SP on the mitochondrial membrane potential in macrophages. Moreover, the seahorse glycolysis stress test was used to detect glycolysis and aerobic oxidation in macrophages. We found that SP-treated macrophages had a higher oxygen consumption rate, and both NK-1 inhibitor (RP67580) and SGMS2 inhibitor (LY93) blocked the elevation in oxygen consumption rate (Figure 5I), suggesting that SP/SGMS2 may regulate mitochondrial function. In the seahorse glycolysis stress test, we found that the ECAR level in SP-treated macrophages was not markedly different from that in untreated macrophages (Figure 5J), indicating that SP greatly enhanced mitochondrial function, while glycolysis did not change markedly. In summary, SP induces M2 differentiation via the sphingolipid metabolic pathway, especially SMGM2, which modulates mitochondrial metabolic function and reprograms macrophage metabolism, leading to M2 polarization.
Figure 6|SP promotes neutrophil infiltration and NET formation.
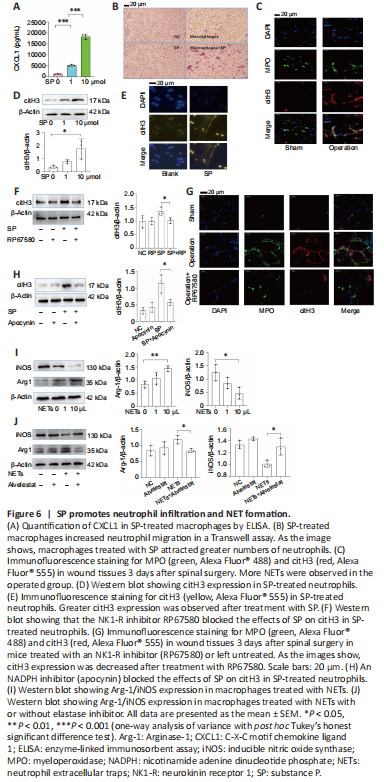
In addition to metabolic reprogramming, we found an indirect pathway through which SP induces M2 differentiation. When macrophages were stimulated with SP, we found that they secreted more CXCL1 (Figure 6A), a neutrophil chemokine (Drummond et al., 2019). In line with the elevated production of CXCL1, SP-treated macrophages promoted neutrophil migration in a Transwell assay (Figure 6B). We previously showed that NETs increase α-SMA and fibronectin production by macrophages (Jin et al., 2020), and indeed, more NETs (as detected by the immunofluorescence detection of the biomarker citH3) were observed at the injury site in operated mice than in sham mice (Figure 6C). Various stimuli can evoke NET production. We next asked whether SP is involved in NET production. As expected, SP increased the expression of citH3, a biomarker for NETs (Park et al., 2020) (Figure 6D). Moreover, a fibrous extracellular structure that stained positively for citH3 was observed surrounding SP-stimulated neutrophils (Figure 6E). Inhibiting SP with RP67580 diminished citH3 production in vitro (Figure 6F) and NET formation in vivo (Figure 6G), further suggesting that SP directly triggers NET formation. NADPH oxidase is a key factor in NET generation. When NADPH was inhibited by treatment with apocynin, citH3 expression decreased in SP-treated neutrophils (Figure 6H), indicating that SP induces NET formation through NADPH.
Based on our previous work (Jin et al., 2020), we examined whether SP-induced NETs induce M2 macrophage differentiation. As shown in Figure 6I, SP-induced NETs increased Arg-1 expression but decreased iNOS expression, suggesting that SP-induced NETs may promote M2 differentiation. An elastase inhibitor rescued the changes in Arg-1 and iNOS expression in NET-treated macrophages (Figure 6J), indicating that elastase in NETs may be indispensable for M2 differentiation.
Figure 7|An NK1-R inhibitor (RP67580) alleviates epidural fibrosis.
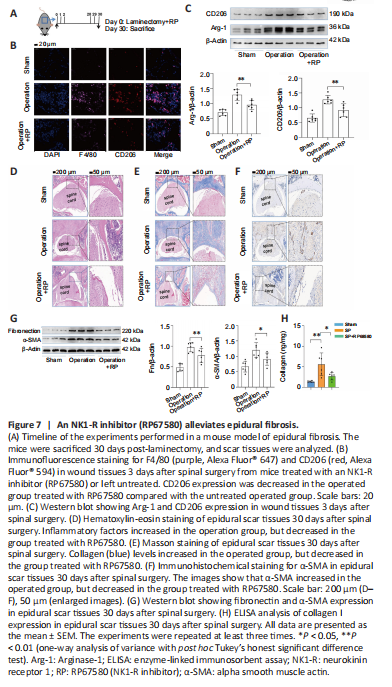
We further tried to block the SP pathway using RP67580 in vivo to lessen epidural fibrosis in a mouse model of laminectomy (Figure 7A). The immunofluorescence results showed that a large number of F4/80+CD206+ cells were recruited to the epidural wound site 3 days after spinal surgery, which was alleviated by treatment with RP67580 (Figure 7B). Moreover, RP67580 treatment decreased Arg-1 and CD206 expression levels in tissue from the injury site (Figure 7C). HE (Figure 7D) and Masson (Figure 7E) staining were performed on the spinal tissues 30 days after spinal surgery. Inflammatory factors and collagen deposition were increased after surgery, and this phenomenon was alleviated by treatment with RP67580. Finally, we used immunohistochemistry, western blotting, and ELISA analysis to detect the expression of α-SMA (Figure 7F and G), fibronectin (Figure 7G), and collagen I (Figure 7H), which suggest the formation of epidural fibrosis. We found that the increased fibronectin, α-SMA, and collagen I expression in the scar tissues caused by surgery could indeed be lessened by blocking the SP receptor by treatment with RP67580. Collectively, these results suggest that inhibiting SP with RP67580 reduced M2 differentiation in the injured tissues and alleviated epidural fibrosis after spinal surgery.