中国神经再生研究(英文版) ›› 2023, Vol. 18 ›› Issue (10): 2252-2259.doi: 10.4103/1673-5374.369120
P物质通过诱导巨噬细胞的M2分化促进脊髓硬膜外纤维化
Substance P promotes epidural fibrosis via induction of type 2 macrophages
Feng Hua1, #, Hao-Ran Wang1, #, Yun-Feng Bai1, Jin-Peng Sun1, Wei-Shun Wang2, Ying Xu3, Ming-Shun Zhang4, *, Jun Liu1, *
- 1Department of Orthopedics, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China; 2Department of Orthopedics, Sir Run Run Hospital, Nanjing Medical University, Nanjing, Jiangsu Province, China; 3Department of Intensive Care Unit, Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China; 4NHC Key Laboratory of Antibody Technique, Jiangsu Province Engineering Research Center of Antibody Drug, Department of Immunology, Nanjing Medical University, Nanjing, Jiangsu Province, China
摘要:
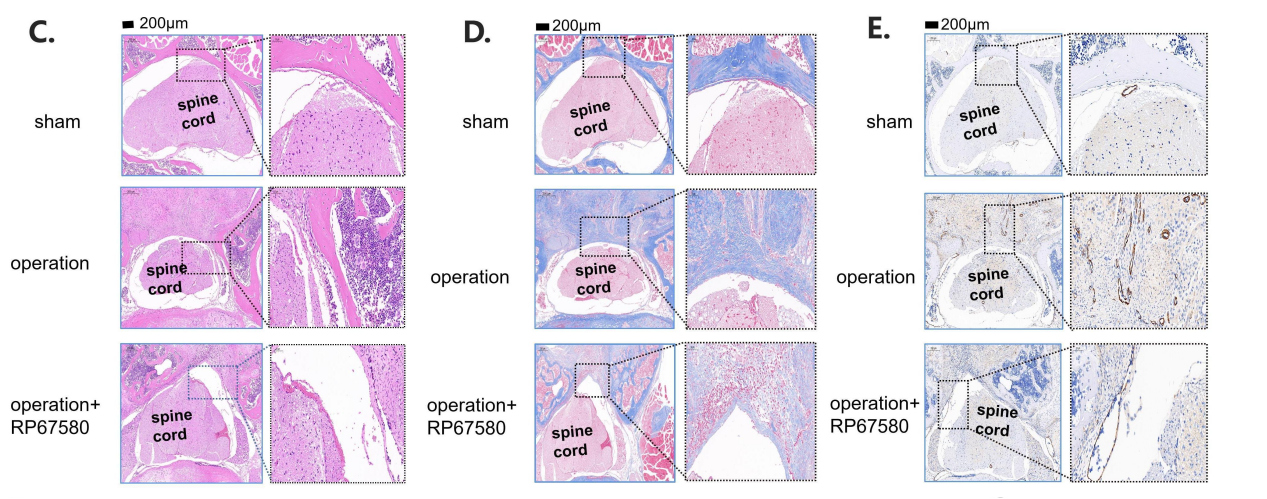
脊柱手术后,神经元会向硬膜外部位分泌大量P物质,而后者与巨噬细胞分化和瘢痕纤维化疾病有关,但是P物质在硬膜外瘢痕纤维化中的具体作用和机制仍不清楚。为此,实验构建了L1-L3椎板切除小鼠模型,可见背根神经节神经元以及伤口部位浸润的巨噬细胞能释放P物质。进而在体外实验中发现,M1巨噬细胞分泌的P物质能促进M1巨噬细胞向M2巨噬细胞的分化。接下来的高通量mRNA测序结果表明,鞘脂代谢通路可能参与P物质调节M2分化。具体而言,鞘磷脂代谢通路中的鞘磷脂合成酶2可促进P物质调节巨噬细胞的M2分化。且鞘磷脂合成酶2抑制剂LY93抑制鞘磷脂合成酶2可抑制M2分化。此外,P物质还能促进中性粒细胞胞外陷阱网的形成,进一步促进M2分化。最后在椎板摘除小鼠模型中发现,P物质受体抑制剂RP67580可显著减少伤口部位M2巨噬细胞,减轻硬膜外瘢痕,并降低瘢痕组织中纤维连接蛋白、α-平滑肌肌动蛋白和I型胶原含量。上述结果表明,P物质可通过鞘磷脂合成酶2和中性粒细胞胞外陷阱网促进硬膜外纤维化中巨噬细胞的M2分化,这将为治疗硬膜外瘢痕提供了新的策略。
https://orcid.org/0000-0001-5925-0168 (Ming-Shun Zhang); https://orcid.org/0000-0003-3756-3627 (Jun Liu)